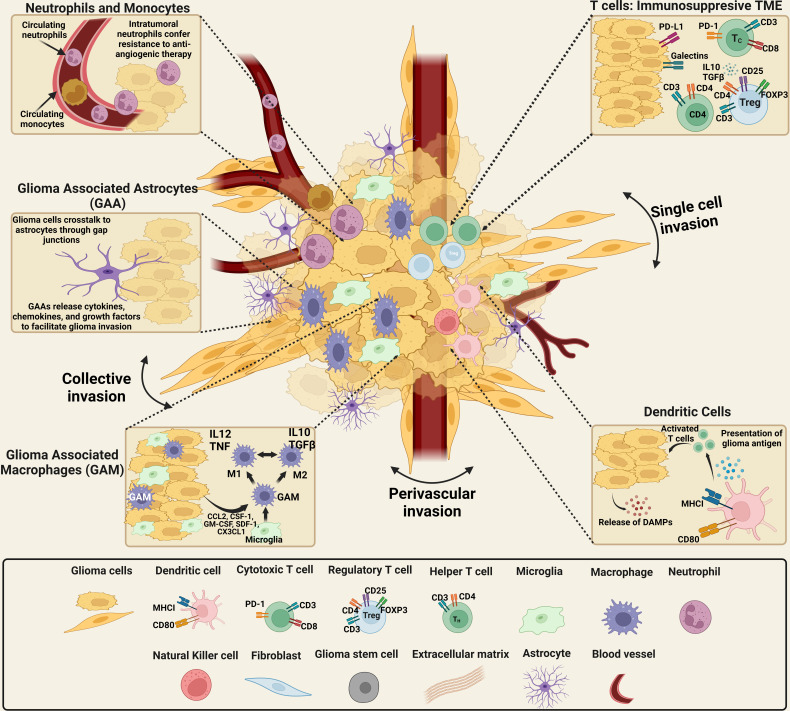
Figure 1.
Schematics of the cellular components of the brain tumor microenvironmental landscape. The cellular components of gliomas: malignant and non-malignant cells, including tumor cells, a range of invasive peripheral immune cells, cells from the healthy brain including neurons and neuroglia, as well as pericytes and endothelial cells. The non-malignant cellular component consists of local immune cell types, including microglia and astrocytes, as well as lymphocytes, endothelial cells. High numbers of neutrophils in the systemic circulation indicates a positive therapeutic response. However, the presence of neutrophils in the glioma microenvironment confers resistance to anti-angiogenic therapy, suggesting high-grade glioma. Neutrophils promote the proliferation of GSCs with mesenchymal phenotypes and GSCs recruited through TGF-β secretion induce an immunosuppressive and, protumoral, M2 phenotype in macrophages. TGF-β and IL-10, immunosuppressive cytokines that impair T-cell response and antigen-presenting cell function, are overexpressed by glioma cells. The expression of MHC-I is downregulated in glioma cells while PD-L1 is upregulated, impairing T-cell response. Additionally, CTLA-4 expression reduces TCR activity. Recruitment and development of regulatory T cells are stimulated by TGF- and IL-10. Finally, the release of GM-CSF and CSF-1 by glioma cells promotes the recruitment of macrophages and polarization of M1 macrophages to an M2-like phenotype. The tumor–brain interface is distinguished by an invasive edge that harbors invasive glioma cells that migrate via white matter tracts or extracellular matrix fibers to infiltrate the brain parenchyma either collectively or as a single cell invasion. Glioma cells have been demonstrated to invade the perivascular space collectively, as a conduit for invasion. [Created with BioRender.com].