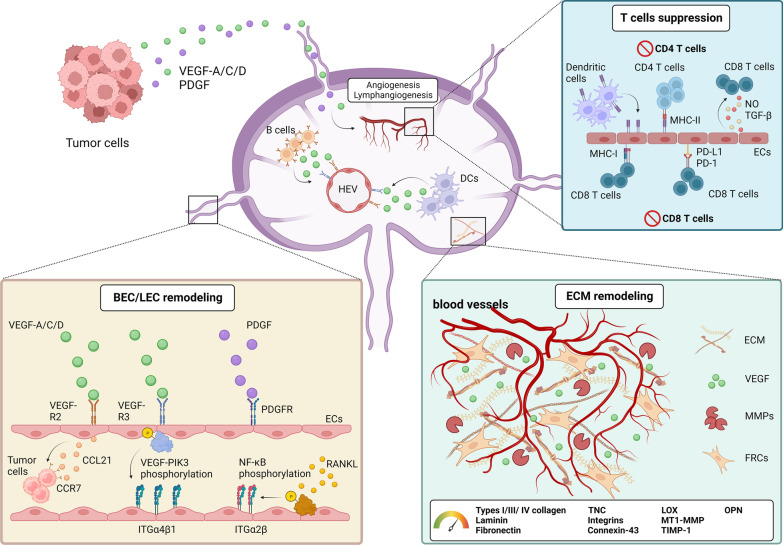
Fig. 2.
Endothelial cell reprograming in the SLN during cancer progression. Tumor cell-derived VEGF and PDGF directly activate their specific receptors on LECs. Signals induce ITGα4β1 expression via the VEGFR-3/PI3K axis. VEGFR-3 activation also contributes to CCL21 production by LEC, further attracting CCR7-expressing tumor cells homing to the SLN. PDGF from tumor cells stimulates the proliferation of LEC, providing more opportunities for tumor cells to establish the pre-metastatic niche. LEC activation by RANKL in the SLN enhances ECM remodeling to trigger the sprouting of LEC and BEC to enhance tumor metastasis to secondary lymphoid organ. B cells and DCs also secrete VEGF-A to remodel the BECs in HEV, leading to the decrease in vessel wall and the increase in HEV diameter which facilitate tumor cell metastasis and provide nutrient for sustaining tumor cell growth in the SLN. MHC II complexes acquired from DC present on cell surface of LEC induces apoptosis of CD4+ T cells. CD8+ T cell proliferation is also suppressed by the PD-L1 molecule expressed on LEC. Moreover, LEC can generate soluble factors such as nitric oxide and TGF-β to inhibit CD8+ T cell activation