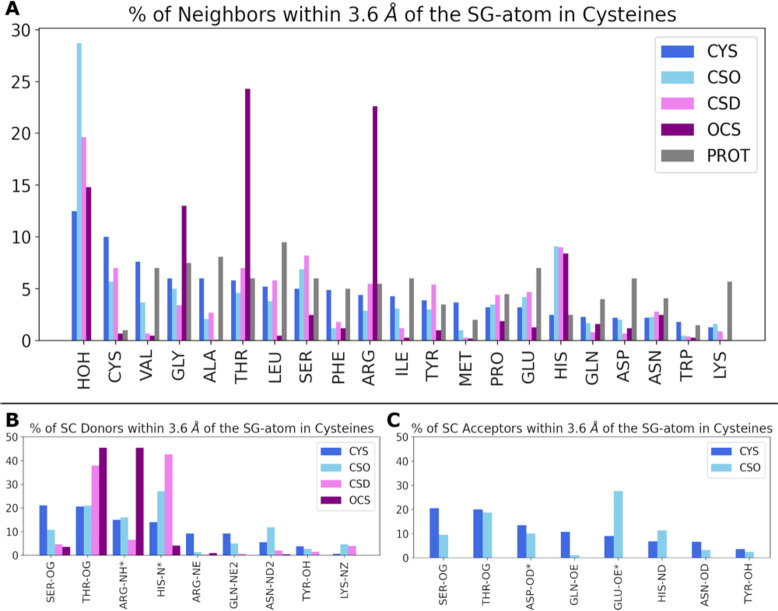
Figure 2.
Local environment of different cysteine side chain oxidation states. (A) Neighboring amino acids (and crystallographic waters) within a cutoff of 3.6 Å around the sulfur atom (SG), compared to the average abundance of amino acids from proteins in the Protein Data Bank (“PROT”, gray).53 The amino acids are arranged along the x-axis in decreasing order for the CYS distribution; i.e., an unmodified CYS is most likely to be found near a crystallographic water or another CYS, and least likely to be found near Trp or Lys. (B) Side chain hydrogen bond donors within 3.6 Å of the sulfur. (C) Side chain hydrogen bond acceptors within 3.6 Å of the sulfur. Data for hydrogen bonds involving backbone amide groups are presented in Figure S3.