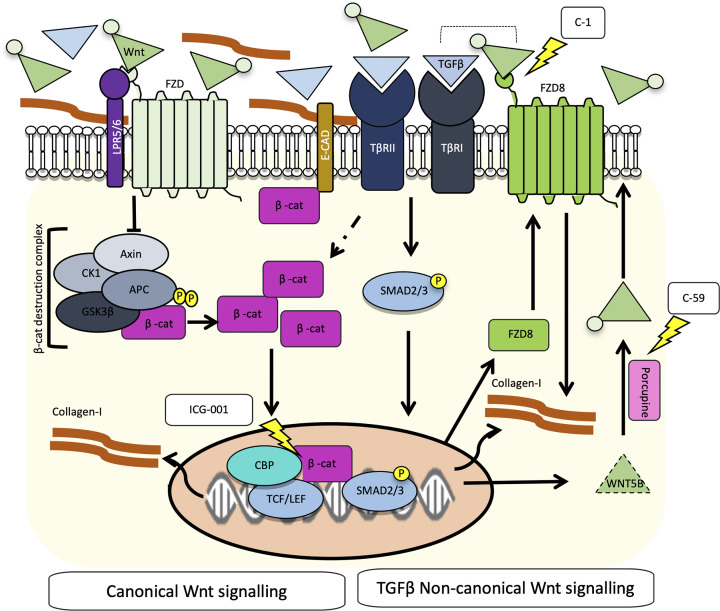
Figure 7. Model for Wnt-mediated fibrosis in CD.
Binding of Wnt ligands to FZD receptors leads to canonical activation, inhibition of the β-catenin destruction complex, and an increase in dephosphorylated active β-catenin. β-catenin is then free to translocate to the nucleus, where along with cofactors such as CBP, it activates TCF/LEF-dependent gene transcription and as shown in the present study up-regulates Collagen-I. Conversely, inhibitors of the β-catenin-dependent transcription such as ICG-001 reduce Collagen-I expression. ICG-001 can also disrupt interactions between Smad3 and β-catenin are CBP-dependent [34]. In intestinal fibroblasts, the profibrotic cytokine TGFβ does not directly activate the canonical Wnt pathway in intestinal fibroblasts. Instead, TGFβ promotes noncanonical Wnt signalling mediated by FZD8/Wnt5B. Inhibiting either the FZD8 receptor with a small-molecule inhibitor (C1; 3235-0367) or blocking Wnt ligand production in TGFβ-stimulated fibroblasts, which also results in reduced Collagen-I expression from intestinal fibroblasts. These two parallel pathways can regulate Collagen-I independently but there is also potential for cross-talk, given that the TGFβR complex can associate with the FZD8 receptor and TGFβ1 can promote the accumulation of β-catenin in fibroblasts, which may prime cells to be more Wnt-responsive.