Extended Data Figure 10: Temporal and permanent blockades of NTSR1 signal in the BLA produces the different behavioral effects.
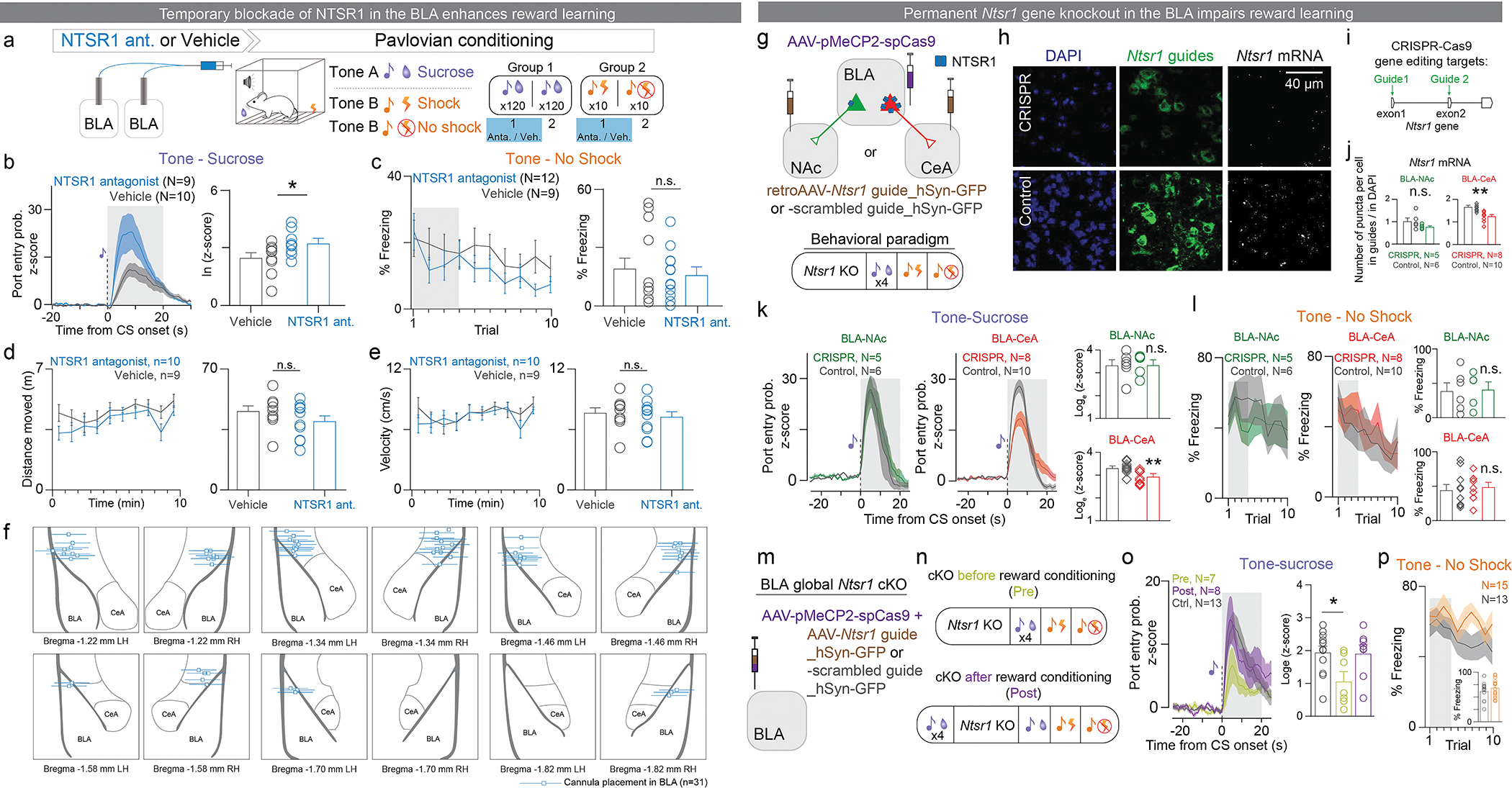
a-f: Temporal inhibition of NTSR1 in the BLA enhanced tone-sucrose association. (a) Experimental design to test the role of NTSR1 in the BLA during reward and punishment learning. Mice received a bilateral intracranial injection locally into the BLA of either NTSR1 antagonist (SR48692) or vehicle 15–20 minutes before the first session of Pavlovian sucrose or shock conditioning. Mice were reward conditioned by pairing a tone with a sucrose reward 120 times per session over two sessions, and punishment conditioned by pairing a different tone with a footshock 10 times in one session. (b) Normalized population average for the entire 120 pairings of session 2 (Shaded region indicates the time window when the peak was selected; Unpaired t-test, t16=2.128, *P=0.049, effect size = 0.7431 ± 0.3491, CI95=0.0029 to 1.483). (c) Population data quantifying percentage of freezing during the tone (Unpaired t-test, t19=1.061, P=0.3021, effect size=7.785 ± 7.339, CI95=−7.577 to 23.15). (d) Distance moved in a 10-minute OFT by mice receiving NTSR1 antagonist or vehicle bilaterally into the BLA (left). Total distance travelled in 10 minutes did not differ between mice treated with intra-BLA NTSR1 antagonist or vehicle (right; unpaired t-test, two-tailed, t17=1.212, P=0.2420, effect size= 5.569 ± 4.594, CI95= −4.124 to 15.26). (e) Velocity in the open field (left). The average rate of movement in the OFT did not differ between mice treated with intra-BLA NTSR1 antagonist vs. vehicle (right; unpaired t-test, two-tailed, t17=0.5012, P=0.6227, effect size= 0.3852 ± 0.7686, CI95= −1.237 to 2.007). (f) Placement of bilateral cannula tips above the BLA for delivery of NTSR1 antagonist in mice tested in sucrose and shock learning paradigms.
g-p: Permanent Ntsr1 gene CRISPR-cKO in the BLA impaired tone-sucrose association. (g) Schematic of viral injections. Retrograde AAV encoding Ntsr1 guide RNAs or the negative control guide were injected bilaterally in the NAc or the CeA and AAV5 or AAV9 encoding Cas9 was injected bilaterally in the BLA. (h) Representative confocal images of DAPI, Ntsr1 guide positive cells, and NRSR1 mRNA in situ hybridization. (i) Ntsr1 guide RNAs were designed to target exon1 and exon2 of the Ntsr1 gene. (j) Ntsr1 mRNA levels in BLA-CeA neurons is significantly reduced in the CRISPR group compared to the control group (Unpaired t-test, two-tailed, t16=4.503, ***P=0.0004, effect size= −0.3844 ± 0.0853, CI95= −0.5654 to −0.2035). There is no difference in Ntsr1 mRNA levels in BLA-NAc neurons, possibly due to the small amount of basal Ntsr1 signal in BLA-NAc neurons (Unpaired t-test, two-tailed, t9=1.629, P=0.1377, effect size= −0.2457 ± 0.1508, CI95= −0.5869 to 0.00945). mRNA levels were calculated as the ratio of the average number of mRNA puncta in guide-positive neurons to DAPI-positive neurons. (k) Ntsr1 CRISPR cKO in BLA-CeA neurons, but not in BLA-NAc neurons reduced port entry probability in response to sucrose-predictive cues (Unpaired t-test, two-tailed, BLA-CeA, t16=3.577, **P=0.0025, effect size= −0.4462 ± 0.1248, CI95= −0.7107 to −0.1818, BLA-NAc, t9=0.03604, P=0.972, effect size= 0.01156 ± 0.3208, CI95= −0.7141 to 0.7373). (l) Ntsr1 cKO in either BLA-CeA or BLA-NAc neurons affected tone-shock recall test (Unpaired t-test, two-tailed, BLA-NAc, t9=0.1234, P= 0.9045, effect size= 0.02031 ± 0.1646, CI95= −0.3520 to 0.3927; BLA-CeA, t16=0.4075, P=0.689, effect size= 0.04435 ± 0.1088, CI95= −0.1864 to 0.2751). (m) Schematic of viral injections of global BLA Ntsr1 CRISPR-cKO. (n) The BLA global Ntsr1 CRISPR-cKO was performed before or after the acquisition of tone-sucrose association in separate groups. (o) The BLA global Ntsr1 CRISPR-cKO prior to the acquisition impaired subsequent tone-sucrose association, while the CRISPR-cKO post the acquisition did not affect the memory recall (One-way ANOVA, F(2,25)= 4.324, *P= 0.024. Holm-Sidak’s multiple comparisons test, Control vs. Pre: *P= 0.019; Control vs. Post: P= 0.99). (q) The CRISPR-cKO globally in BLA neurons did not affect tone-shock association (Unpaired t-test, two-tailed, t26=0.871, P=0.3917, effect size= 0.05526 ± 0.06345, CI95= −0.07516 to 0.1857).
N denotes number of mice in each group. Error bars and solid shaded regions around the mean indicate s.e.m.
