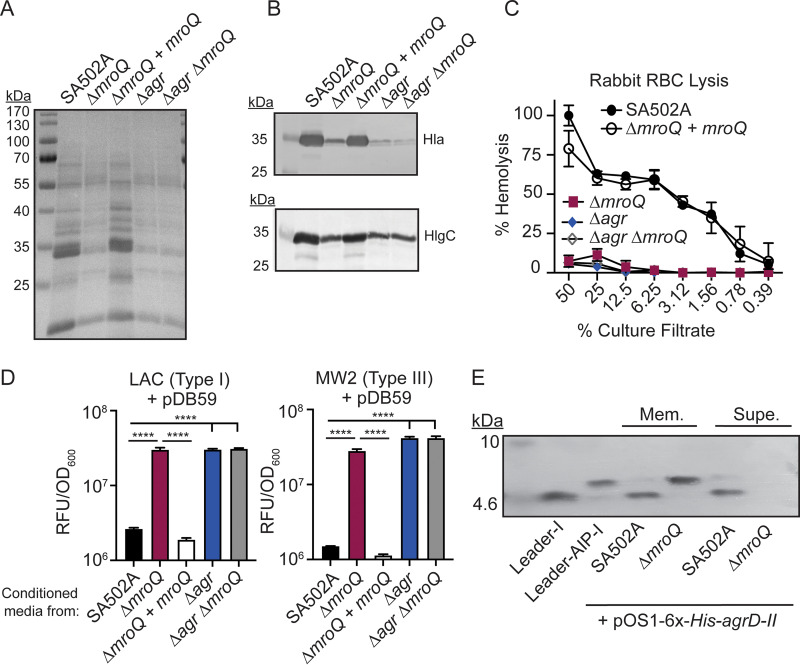
FIG 4.
A ΔmroQ mutant is defective for Agr type II activation and AIP-II maturation and export. (A) TCA-precipitated exoproteins from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (B) Hla and HlgC immunoblots from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (C) Rabbit red blood cell lysis of cell-free culture filtrates derived from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (D) pDB59 reporter activity (RFU/OD600) of LAC (left) and MW2 (right) upon addition of conditioned medium from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (E) Immunoblots of supernatant and membrane fractions from SA502A and ΔmroQ strains constitutively expressing 6×-His-AgrD-II (pOS1-PsarA-6×-His-agrD-II) using anti-His monoclonal antibody. 6×-His-leader-AIP-I (AgrB processing intermediate) and 6×-His-leader-I (AgrD-I leader peptide) were isolated from constitutively expressing S. aureus and were included as controls. Hemolysis and reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots and GelCode blue-stained gels are representative of at least four replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by one-way ANOVA with Tukey’s posttest.