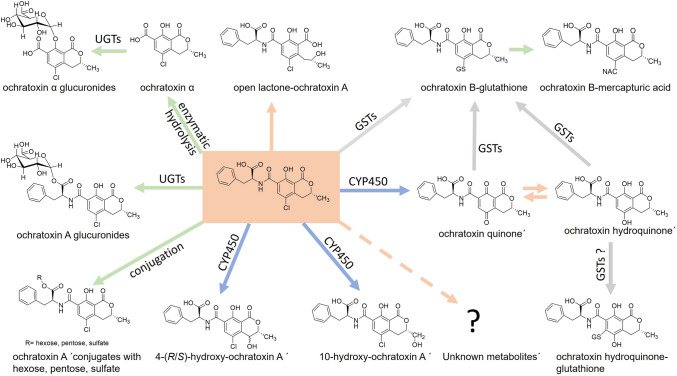
Fig. 1.
Biotransformation of ochratoxin A (OTA; central box) to metabolites identified in vivo and/or in vitro and proposed intermediates. Some pathways (green arrows) result in the formation of a product (OT-alpha) with relatively low toxicity and to conjugated metabolites which are more readily excreted than the parent mycotoxin. Formation of hydroxylated OTA metabolites by CYP450 enzymes (blue arrows) is also regarded as detoxication reaction. In contrast, CYP-mediated oxidation of OTA to quinone/hydroquinone intermediates (pink arrows) is considered as potential bioactivation reaction. The quinone/hydroquinone couple may react with cellular macromolecules or undergo conjugation by GSTs (grey arrows) to glutathione conjugates, e.g., the OTB-GSH metabolite which is then transformed into a mercapturic acid. Opening of the lactone ring of OTA (pink arrow to the top) yields a product with increased toxicity. See text for further details and references on OTA metabolism. A pink arrow pointing to a question mark denotes hypothetical as yet unknown bioactivation reactions for OTA