Abstract
Objective
The Phase 3 DISCOVER-1 study of guselkumab is the first randomized controlled trial to use Patient-Reported Outcomes Measurement Information System (PROMIS) measures to assess the effects of treatment on general health outcomes in patients with psoriatic arthritis (PsA).
Methods
Patients (N = 381) with active PsA were randomized 1:1:1 to guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at Week 0, Week 4, then every 8 weeks (Q8W); or placebo with Week 24 crossover to guselkumab Q4W. The PROMIS-29 Profile contains four items for each of seven domains (anxiety, depression, fatigue, pain interference, physical function, sleep disturbance, and social participation) and one pain-intensity item. Raw domain scores are converted to standardized T-scores, with norms based on a US general population mean of 50 (1 standard deviation (SD) = 10). T-score changes of ≥ 5 are considered clinically meaningful. Least-squares mean PROMIS-29 T-score changes from baseline to Week 24 and Week 52 were summarized for the guselkumab and placebo groups; nominal p-values comparing results between guselkumab and placebo were calculated at Week 24 using a mixed model for repeated measures. The proportions of patients who achieved clinically meaningful improvement in PROMIS-29 T-scores were also summarized at Week 24 and Week 52; nominal p-values comparing results between guselkumab and placebo were calculated at Week 24 using the Cochran-Mantel-Haenszel test.
Results
In the DISCOVER-1 patient population, mean PROMIS-29 T-scores at baseline were ~ 1 SD worse for physical function and pain interference and were numerically worse for social participation, fatigue, and sleep disturbance compared with the US general population. At Week 24, mean PROMIS-29 T-scores improved in guselkumab-treated patients, approaching US population norms; T-scores continued to improve through Week 52. Significantly higher proportions of patients in both guselkumab treatment arms (31–52% across domains) had clinically meaningful improvements in pain interference, fatigue, physical function, sleep, and social participation at Week 24 versus placebo (all nominal p ≤ 0.05).
Conclusion
In patients with active PsA, guselkumab treatment provided clinically meaningful reductions in fatigue and pain and improvement in physical function and social participation, as measured by the PROMIS-29 Profile. These improvements were maintained through 1 year.
ClinicalTrials.gov
Registration number, NCT03162796; Submission date 19 May 2017.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40271-022-00588-6.
Key Points for Decision Makers
| Patient-Reported Outcomes Measurement Information System (PROMIS) measures are increasingly being used in clinical research and in real-world practice to evaluate general health outcomes that affect quality of life in general populations and in patients with chronic diseases. |
| DISCOVER-1 is the first randomized controlled trial to assess health-related quality of life in patients with psoriatic arthritis using PROMIS measures, specifically the PROMIS-29 Profile. |
| PROMIS-29 Profile results from DISCOVER-1 indicate that treatment with guselkumab was associated with clinically meaningful improvements across all health-related quality-of-life domains; higher proportions of patients in both guselkumab treatment arms had clinically meaningful improvements in pain interference, fatigue, physical function, sleep, and social participation at Week 24 versus placebo (all nominal p ≤ 0.05). |
| All mean PROMIS-29 T-scores in the guselkumab groups approached US population norms at Week 24, and mean improvements were maintained through Week 52. |
Introduction
Psoriatic arthritis (PsA) is a chronic, heterogeneous, inflammatory disease that affects approximately 30% of patients with psoriasis [1]. Characteristic musculoskeletal and dermatologic manifestations of PsA include peripheral arthritis, spondylitis, dactylitis, enthesitis, and psoriatic skin and nail lesions [1, 2]. The joint manifestations of PsA often cause symptoms of pain, swelling, tenderness, and reduced mobility, and skin lesions can be painful, pruritic, and irritating [3].
Beyond these central joint and skin features of PsA, patients frequently experience a broad range of other symptoms that negatively impact their health-related quality of life. The chronic, often debilitating nature of PsA has been shown to affect virtually all aspects of patients’ lives, including the domains of physical and emotional functioning, social participation, fatigue, sleep, coping, intimacy, self-care, depression, stress, and work productivity [3–5]. A recent global survey (N = 1286) found that despite taking prescription medications for the treatment of PsA, more than half of all patients with the disease reported moderate or major negative impacts on their level of physical activity, ability to perform certain activities, work productivity, emotional/mental well-being, romantic relationships and intimacy, and participation in social or recreational activities, and 52% of patients reported experiencing unusual fatigue [6].
As awareness of the broad life impact of PsA has increased, consensus guidelines have been updated to recommend that all late-stage clinical trials measure outcomes identified as being important to patients, including the effects of treatment on fatigue, physical function, pain, emotional well-being, and social participation [5, 7]. The Patient-Reported Outcomes Measurement Information System (PROMIS®) is a set of self-reported measures developed by the National Institutes of Health to evaluate physical, mental, and social health in general populations and in patients with chronic diseases [8–10]. The PROMIS-29 Profile includes 29 items assessing the following seven domains of health: physical function, pain interference, fatigue, sleep disturbance, satisfaction with participation in social roles and activities, depression, and anxiety; plus a single pain-intensity numeric rating scale (NRS) [11]. Validated PROMIS Profile measures are increasingly being used in clinical research and in real-world practice to evaluate general health outcomes that affect quality of life, especially in patients with rheumatic and musculoskeletal diseases [11–19].
Guselkumab, a fully human monoclonal antibody that selectively binds and inhibits the p19 subunit of interleukin-23, is approved for the treatment of adults with active PsA, as well as moderate to severe plaque psoriasis [20]. Results from the pivotal randomized, placebo-controlled, Phase 3 DISCOVER-1 study showed that guselkumab 100 mg administered every 4 weeks (Q4W) or every 8 weeks (Q8W) significantly improved joint symptoms, physical function, and skin symptoms of psoriasis in patients with active PsA [21, 22]. Here we report the treatment effects of guselkumab on health-related quality of life in patients with active PsA in the DISCOVER-1 study based on PROMIS-29 Profile results through 1 year.
Methods
Full details of the DISCOVER-1 study design and key clinical efficacy and safety results have been reported previously [21, 22]. This randomized, double-blind, placebo-controlled study (NCT03162796) was initiated on 28 August 2017, and the last study visit was 14 November 2019. The study was conducted at 86 sites in 13 countries (Australia, Canada, Czech Republic, Germany, Hungary, Malaysia, Poland, South Korea, Russia, Spain, Taiwan, Ukraine, and the USA).
Study Population
Eligible participants had active PsA despite standard therapies (non-biologic disease-modifying anti-rheumatic drugs (DMARDs), apremilast, and non-steroidal anti-inflammatory drugs (NSAIDs)), and a current or documented history of psoriasis with inadequate response or intolerance to standard treatments. Active PsA was defined as having at least three swollen and three tender joints, and C-reactive protein concentration ≥ 0.3 mg/dL. Approximately 30% of enrolled patients could have had previous exposure to one or two tumor necrosis factor inhibitors.
Study Design
Participants were randomized 1:1:1 to receive guselkumab 100 mg Q4W; guselkumab 100 mg at Week 0, Week 4, then Q8W; or placebo with crossover to guselkumab 100 mg Q4W at Week 24. The study consisted of a 6-week screening period, a 24-week placebo-controlled period, and a 24-week active treatment period, with safety measurements through Week 60. At Week 16, patients with < 5% improvement in both swollen and tender joint counts were eligible for early escape; i.e, these patients continued with study treatment, but the investigator could initiate or increase their dose of NSAIDs or other analgesics, oral corticosteroids (≤ 10 mg/day of prednisone or equivalent), or nonbiologic DMARDs [21, 22].
PROMIS-29 Assessments
The PROMIS-29 Profile (v2.0) was administered at baseline and at the Week 8, 16, 24, 36, and 52 study visits. The PROMIS-29 Profile is a set of generic short forms containing four items for each of the seven PROMIS domains (scored from 1 to 5), along with a single pain-intensity item (scored on a 0–10 NRS). Patients report anxiety, depression, fatigue, sleep disturbance, pain interference, and pain intensity over the past 7 days; physical function and ability to participate in social roles and activities have no specified timeframe. Raw scores for all domains (except pain-intensity NRS) are converted into standardized T-scores with a mean of 50 and a standard deviation (SD) of 10. For the depression, anxiety, physical function, pain interference, and fatigue domains, a score of 50 represents the average for the US general population. The centering samples for the sleep disturbance and satisfaction with social roles and activities domains are a combination of a US general population and a clinical sample. For these domains, a score of 50 likely represents somewhat sicker people than the US general population. Higher PROMIS T-scores represent more of the concept being measured. For the anxiety, depression, fatigue, pain interference, and sleep disturbance domains, higher scores indicate more severe symptoms. For the physical function and social participation domains, higher scores indicate better health outcomes [8].
Statistical Analyses
All statistical analyses were performed using SAS software. Least-squares mean PROMIS-29 T-score changes from baseline to Week 24 and Week 52 were summarized by treatment group. Least-squares mean changes in T-scores from baseline were calculated using observed data; at Week 24, the change from baseline was set to zero (no change) if a patient met treatment failure criteria (i.e., discontinued study treatment, terminated study participation, initiated or increased DMARD or oral corticosteroid use, or initiated protocol-prohibited PsA treatment); at Week 52, the change from baseline was set to zero if data were missing after patients discontinued the study agent for any reason. A mixed model for repeated measures was used to calculate least-squares mean changes in T-scores for the placebo and guselkumab groups at Week 24 and Week 52 and to calculate nominal p-values (not adjusted for multiplicity) between guselkumab and placebo at Week 24; comparisons between guselkumab and placebo were not performed after patients in the placebo group crossed over to guselkumab Q4W at Week 24.
Modified cumulative distribution curves [23] were generated to show the proportions of patients achieving various levels of improvement in each PROMIS-29 domain T-score from ≥ 1 to ≥ 21 points at Week 24 by treatment group. Based on published studies estimating clinically meaningful changes and minimally important differences in PROMIS domain scores in patients with rheumatic and musculoskeletal disorders [15, 24–27], two prespecified thresholds (≥ 5- and ≥ 3-point improvement) were used to define clinically meaningful changes in each domain T-score. At Week 24, achievement of point improvement thresholds was calculated for all patients who had not met treatment failure criteria; patients with missing data were considered non-responders. At Week 52, proportions of patients achieving ≥ 5- or ≥ 3-point improvements were calculated for patients who remained in the study; patients who discontinued the study agent for any reason and had missing data were considered non-responders. At Week 24, nominal p-values (not adjusted for multiplicity) were calculated for the difference in the proportion of patients achieving ≥ 5- or ≥ 3-point improvements in the guselkumab versus placebo groups using the Cochran-Mantel-Haenszel test.
Effect sizes for PROMIS-29 T-score changes from baseline to Week 24 and Week 52 were calculated (using observed data) as the mean value of change from baseline divided by the SD of the baseline value [28]. Effect size absolute values of ≤ 0.2 are considered trivial; values of > 0.2 to < 0.5 are considered small; values of 0.5 to < 0.8 are considered moderate, and values ≥ 0.8 are considered large [29].
Results
Patient Characteristics
Patients in the DISCOVER-1 study had established and active disease, with an average of nine to 11 swollen joints, 18 to 20 tender joints, and moderate pain at baseline (Table 1). Baseline characteristics were generally comparable across treatment groups. Baseline PROMIS-29 T-scores indicate that on average, patients in this study had significantly impaired physical function and more intense and impactful pain (~ 1 SD worse) relative to the general US population. For the domains of fatigue, sleep disturbance, and social participation, mean baseline T-scores were numerically worse than US population norm value of 50, but were within 1/2 SD (5 points) of the norm. For the domains of anxiety and depression, mean baseline scores were close to the US population norm of 50.
Table 1.
DISCOVER-1 baseline demographic and disease characteristics
| Characteristica | GUS Q4W (N = 128) | GUS Q8W (N = 127) | Placebo (N = 126) |
|---|---|---|---|
| Age, years | 47.4 (11.6) | 48.9 (11.5) | 49.0 (11.1) |
| Female, n (%) | 62 (48) | 59 (46) | 65 (52) |
| Weight, kg | 86.7 (17.7) | 86.3 (20.0) | 85.2 (18.8) |
| Body mass index, kg/m2 | 29.9 (5.5) | 29.9 (6.4) | 29.6 (5.7) |
| PsA duration, years | 6.6 (6.3) | 6.4 (5.9) | 7.2 (7.6) |
| Number of swollen joints, 0–66 | 8.6 (5.8) | 10.9 (9.3) | 10.1 (7.1) |
| Number of tender joints, 0–68 | 17.7 (13.1) | 20.2 (14.5) | 19.8 (14.4) |
| Patient assessment of pain, 0–10 cm VAS | 5.9 (2.0) | 6.0 (2.1) | 5.8 (2.2) |
| Patient global assessment of arthritis, 0–10 cm VAS | 6.1 (2.0) | 6.5 (2.0) | 6.1 (2.2) |
| HAQ-DI score, 0–3 | 1.1 (0.7) | 1.2 (0.6) | 1.2 (0.7) |
| C-reactive protein, mg/dLb | 0.6 (0.3-1.3) | 0.7 (0.4-1.9) | 0.8 (0.3-1.5) |
| Body surface area of psoriasis, % | 15.0 (18.3) | 13.1 (17.7) | 12.0 (16.0) |
| PASI score, 0–72 | 9.5 (10.1) | 8.4 (9.8) | 7.7 (8.9) |
| Patients with enthesitis, n (%) | 73 (57) | 72 (57)c | 77 (61) |
| Leeds enthesitis index score, 1–6d | 3.0 (1.5) | 2.7 (1.6) | 2.8 (1.6) |
| Patients with dactylitis, n (%) | 38 (30) | 49 (39) | 55 (44) |
| Dactylitis score, 1–60e | 9.4 (12.5) | 8.2 (10.1) | 6.6 (7.4) |
| PROMIS-29 domain (score range)a | |||
| Higher scores indicate more severe symptoms | |||
| Anxiety (40.3–81.6) | 51.9 (9.5) | 52.8 (9.4) | 50.9 (8.7) |
| Depression (41.0–79.4) | 50.4 (8.1) | 51.6 (9.4) | 49.7 (8.6) |
| Fatigue (33.7–75.8) | 53.0 (9.1) | 53.7 (9.6) | 54.2 (8.7) |
| Pain interference (41.6–75.6) | 60.2 (6.7) | 61.8 (6.0) | 61.7 (6.1) |
| Sleep disturbance (32.0–73.3) | 52.0 (6.9) | 52.3 (6.8) | 51.8 (7.3) |
| Pain intensity (0–10 cm VAS) | 6.2 (1.9) | 6.4 (1.9) | 6.3 (2.0) |
| Higher scores indicate better health outcomes | |||
| Physical function (22.9–56.9) | 40.1 (6.7) | 39.5 (7.0) | 39.7 (6.8) |
| Social participation (27.5–64.2) | 47.8 (8.1) | 46.0 (8.4) | 46.9 (8.6) |
aData are mean (SD) unless otherwise stated
bMedian (interquartile range)
cn = 126
dGUS Q4W, n = 73; GUS Q8W, n = 72; placebo, n = 77
eGUS Q4W, n = 38; GUS Q8W, n = 49; placebo, n = 55
GUS guselkumab, HAQ-DI Health Assessment Questionnaire-Disability Index, PASI Psoriasis Area and Severity Index, PROMIS Patient-Reported Outcomes Measurement Information System, PsA psoriatic arthritis, Q4W every 4 weeks, Q8W every 8 weeks, VAS visual analog scale
PROMIS-29 T-Score Changes Over Time
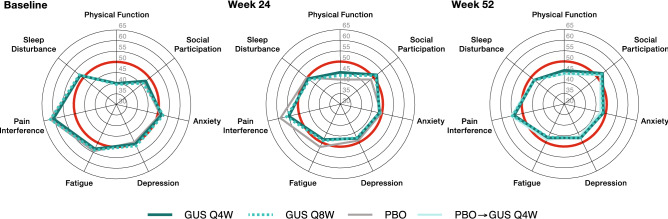
For patients who received treatment with guselkumab, observed mean T-scores for all seven PROMIS-29 domains improved from baseline to Week 24 and Week 52 (Fig. 1). By Week 24, mean T-scores approached US population norm values of 50. By Week 52, absolute improvements in mean T-scores continued to approach, and in some cases exceed, population norm values of 50. In all guselkumab treatment groups (Q4W, Q8W, and placebo crossover to Q4W) at Week 52, mean T-scores for fatigue, anxiety, depression, sleep disturbance, and social participation were numerically better than the US population norm of 50; in guselkumab-randomized patients, mean scores for physical function and pain interference improved to within ~ 0.5 SD of the US population norm.
Fig. 1.
Mean PROMIS-29 T-scores (observed data). The red circles indicate US population norm values of 50 for all domains. For the anxiety, depression, fatigue, pain interference, and sleep disturbance domains, higher scores indicated more severe symptoms. For the physical function and social participation domains, higher scores indicate better health outcomes. GUS guselkumab, PBO placebo, PROMIS Patient-Reported Outcomes Measurement Information System, Q4W every 4 weeks, Q8W every 8 weeks
Across PROMIS-29 domains, least-squares mean T-score changes from baseline in guselkumab-treated patients were numerically, and in most cases nominally significantly, better compared with placebo at Week 24 (p ≤ 0.05 for both guselkumab treatment arms vs. placebo for pain interference, physical function, fatigue, social participation, pain intensity, and depression, and in the guselkumab Q8W treatment arm for sleep disturbance and anxiety) (Table 2). For all PROMIS-29 domains, the improvements observed at Week 24 were maintained or further increased at Week 52 in guselkumab-treated patients.
Table 2.
Least-squares mean (95% CI) changes in T-scores from baselinea
| PROMIS-29 domain (score range) | Week 24 | Week 52 | ||||
|---|---|---|---|---|---|---|
| GUS Q4W (N = 128) | GUS Q8W (N = 127) | Placebo (N = 126) | GUS Q4W (N = 128) | GUS Q8W (N = 124) | Placebo → GUS (N = 123) | |
| A decrease in score indicates reduced severity of symptoms | ||||||
| Anxiety (40.3–81.6) | − 2.9 (− 4.3, − 1.6) | − 3.2 (− 4.6, − 1.9)* | − 1.4 (− 2.7, − 0.0) | − 2.8 (− 4.2, − 1.5) | − 3.4 (− 4.8, − 2.1) | − 3.3 (− 4.7, − 1.9) |
| Depression (41.0–79.4) | − 2.7 (− 3.9, − 1.4)* | − 3.4 (− 4.7, − 2.1)† | − 0.9 (− 2.1, 0.4) | − 2.9 (− 4.1, − 1.8) | − 3.2 (− 4.4, − 2.0) | − 2.5 (− 3.7, − 1.4) |
| Fatigue (33.7–75.8) | − 5.1 (− 6.5, − 3.7)† | − 4.8 (− 6.2, − 3.4)† | − 1.9 (− 3.2, − 0.5) | − 5.7 (− 7.1, − 4.3) | − 6.3 (− 7.7, − 4.9) | − 4.7 (− 6.1, − 3.3) |
| Pain interference (41.6–75.6) | − 5.7 (− 6.9, − 4.5)† | − 5.5 (− 6.7, − 4.3)† | − 2.3 (− 3.5, − 1.1) | − 6.2 (− 7.5, − 5.0) | − 6.0 (− 7.2, − 4.7) | − 5.0 (− 6.2, − 3.7) |
| Sleep disturbance (32.0–73.3) | − 2.5 (− 3.5, − 1.4) | − 3.5 (− 4.6, − 2.4)† | − 1.2 (− 2.3, − 0.1) | − 3.7 (− 4.7, − 2.7) | − 3.9 (− 5.0, − 2.9) | − 2.8 (− 3.8, − 1.7) |
| Pain intensity (0–10 cm VAS) | − 2.3 (− 2.7, − 1.9)† | − 2.0 (− 2.4, − 1.6)† | − 0.6 (− 0.9, − 0.2) | − 2.8 (− 3.2, − 2.4) | − 2.4 (− 2.8, − 2.0) | − 2.0 (− 2.4, − 1.6) |
| An increase in score indicates improvement | ||||||
| Physical function (22.9–56.9) | 5.1 (4.0, 6.1)† | 3.9 (2.8, 5.0)† | 1.3 (0.3, 2.4) | 5.8 (4.7, 6.9) | 4.4 (3.3, 5.5) | 3.4 (2.3, 4.5) |
| Social participation (27.5-64.2) | 4.5 (3.3, 5.8)† | 4.9 (3.7, 6.1)† | 1.5 (0.2, 2.7) | 5.4 (4.1, 6.7) | 5.6 (4.3, 7.0) | 4.0 (2.6, 5.3) |
aDefined as the change from baseline in T-score for all domains except pain intensity (change measured on a 0–10 VAS) using observed data. At Week 24, change from baseline was set to zero (if no improvement) if a patient met treatment failure criteria; at Week 52, change from baseline, if missing, was set to zero after a patient discontinued study agent for any reason. Least-squares mean changes and p-values were calculated using a mixed model for repeated measures analysis
Nominal p values vs. placebo: *p ≤ 0.05; †p ≤ 0.01
CI confidence interval, GUS guselkumab, PROMIS Patient-Reported Outcome Measurement Information System, Q4W every 4 weeks, Q8W every 8 weeks, VAS visual analog scale
Cumulative Distribution of Improvements in PROMIS-29 T-Scores at Week 24
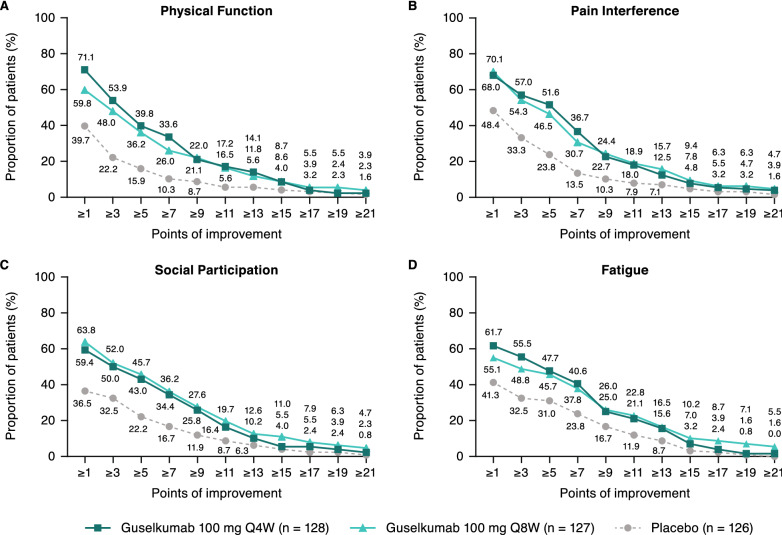
As shown in Fig. 2, clear separation between both guselkumab dosing regimens and placebo was observed over a range of T-score changes from baseline in the PROMIS-29 domains of physical function, pain interference, social participation, and fatigue. More specifically, numerically larger proportions of patients in both the guselkumab Q4W and Q8W groups than in the placebo group achieved ≥ 1- to ≥ 13-point improvements from baseline in all PROMIS-29 domains at Week 24. Clear but smaller separation between both guselkumab dosing regimens and placebo was observed for the PROMIS-29 domains of sleep disturbance, anxiety, and depression (see Supplementary Fig. 1, Online Supplementary Material (OSM)).
Fig. 2.
Cumulative distribution of points of improvement in PROMIS-29 domain physical function (A), pain interference (B), social participation (C), and fatigue (D) T-scores from baseline to Week 24. PROMIS Patient-Reported Outcomes Measurement Information System, Q4W every 4 weeks, Q8W every 8 weeks
Proportions of Patients with ≥ 5- and ≥ 3-Point Improvements
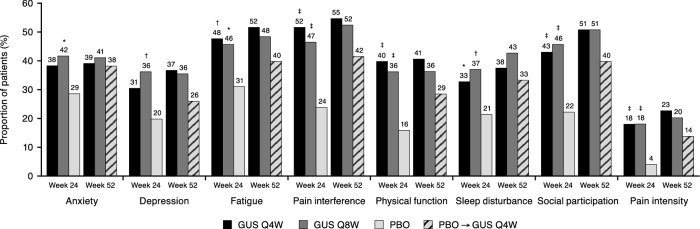
In both guselkumab treatment groups (Q4W and Q8W), greater proportions of patients than in the placebo group had clinically meaningful ≥ 5-point (Fig. 3) and ≥ 3-point (OSM Fig. 2) T-score improvements in all PROMIS-29 domains from baseline to Week 24. The proportions of patients with clinically meaningful improvements afforded by treatment with guselkumab Q4W and Q8W were maintained through Week 52. The proportions of patients in the placebo group achieving clinically meaningful improvement increased after crossover to guselkumab Q4W at Week 24; at Week 52, improvements in this group did not reach the levels achieved by patients who were randomized to guselkumab at baseline.
Fig. 3.
Achievement of ≥ 5-point improvements in PROMIS-29 domain T-scores from baseline to Week 24 and 52. Week 24: All patients, including those with imputed data (patients meeting treatment failure prior to Week 24 or with missing data at Week 24 were considered as not achieving ≥5-point improvement); P-values calculated using Cochran-Mantel-Haenszel test; nominal p-values vs. PBO: *p < 0.05; †p < 0.01; ‡p < 0.001. GUS Q4W, n = 128; GUS Q8W, n = 127; PBO, n = 126. Week 52: Evaluable patients; after discontinuation of study agent for any reason, patients with missing data were considered non-responders. GUS guselkumab, PBO placebo, PROMIS Patient-Reported Outcomes Measurement Information System, Q4W every 4 weeks, Q8W every 8 weeks
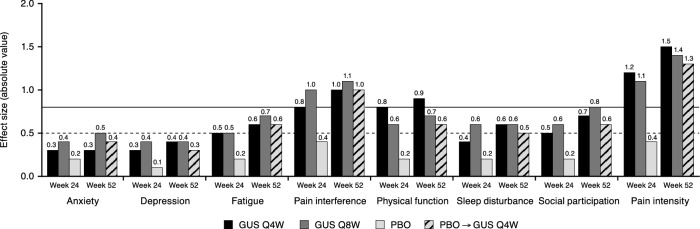
Effect Size
Calculated absolute values of effect sizes for changes from baseline to Week 24 and Week 52 in PROMIS-29 T-scores are shown in Fig. 4. At Week 24, absolute values of effect sizes in guselkumab-treated patients were large (≥ 0.8) for reductions in pain intensity and pain interference and moderate (≥ 0.5) for improvements in physical function, fatigue, and social participation; effect sizes in the placebo group were trivial (0.1–0.2) for anxiety, depression, fatigue, physical function, sleep disturbance, and social participation, and small (0.4) for pain interference and pain intensity. For all domains, the absolute values of effect sizes for changes from baseline at Week 24 were maintained or increased through Week 52. Specifically, in guselkumab-treated patients, absolute values of effect sizes at Week 52 remained large for reductions in pain intensity and pain interference, and were moderate or large for improvements in physical function, social participation, fatigue, and sleep disturbance.
Fig. 4.
Absolute values of effect size for change from baseline in PROMIS-29 T-scores. GUS guselkumab, PBO placebo, PROMIS Patient-Reported Outcomes Measurement Information System, Q4W every 4 weeks, Q8W every 8 weeks
Discussion
Results of this analysis showed that treatment with guselkumab 100 mg Q4W or Q8W was associated with clinically meaningful improvements in health-related quality of life, as measured with the PROMIS-29 Profile. Improvements were observed across all PROMIS-29 domains, most notably pain, fatigue, physical function, satisfaction with social participation, and depression; improvements in these domains were nominally significant for both guselkumab treatment groups compared with placebo at Week 24. Similar to trends observed for the other patient-reported outcome (PRO) measures assessed in DISCOVER-1 and DISCOVER-2 (i.e., Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, Short Form-36 (SF-36) physical component summary (PCS) and mental component summary (MCS) scores, and Health Assessment Questionnaire-Disability Index (HAQ-DI)) [22, 30, 31], mean PROMIS-29 T-score improvements in all domains were maintained or increased from Week 24 to Week 52. Specifically, for the domains of fatigue, sleep disturbance, social participation, physical function, pain interference, and pain intensity, mean T-scores improved from Week 24 to Week 52 in the guselkumab Q4W and Q8W treatment arms, suggesting additional longer-term benefits of treatment, while improvements in anxiety and depression remained stable over time.
Observed effect size absolute values associated with 52 weeks of guselkumab treatment were large (0.8–1.5) for improvements in pain intensity and pain interference; large or moderate (0.6–0.9) for improvements in physical function, social participation, fatigue, and sleep disturbance; and small to moderate (0.3–0.5) for anxiety and depression.
In this analysis, two prespecified thresholds (≥ 5- and ≥ 3-point improvements) were used to define clinically meaningful changes in each PROMIS domain. These thresholds were selected based on conservative estimates of what has been reported in the literature as clinically meaningful or minimally important changes in PROMIS-29 domain scores in patients with rheumatologic or musculoskeletal diseases [15, 24–27, 32, 33]. However, it is noteworthy that reported minimally important differences vary across the different PROMIS domains and across different patient populations. For example, in patients with rheumatoid arthritis, changes as small as 1–2 points are associated with noticeable improvements in domains such as fatigue and pain interference [15, 24, 32], and meaningful change thresholds for these domains have been defined as 2–3 points [34]. Minimal important differences have been reported to be approximately 2 points for PROMIS domains of physical function, fatigue, pain interference, and social participation in patients with systemic lupus erythematosus [33] and to be approximately 2 points for physical function and pain interference in patients with knee osteoarthritis or other chronic musculoskeletal pain [19, 25]. Chen and colleagues found that minimally important differences in pain interference were smaller (1.5–2.5 points) for patients with chronic pain than for a control group of stroke survivors (3–4 points) with baseline pain levels close to the US norm [26]. Thus, in the DISCOVER-1 study population, which had mean baseline pain interference and physical function scores that were approximately 10 points (1 SD) worse than the US general population, it is possible that changes of 1–3 points in these domains may have been clinically meaningful to patients. Cumulative distribution results show that for these domains, 60–71% of guselkumab-treated patients had at least a 1-point improvement and 48–57% had at least a 3-point improvement at Week 24.
Strengths and Limitations
The PROMIS Profile has been extensively studied since its development in 2010, and there is substantial evidence supporting the validity of various PROMIS measures in diverse populations, including patients with rheumatologic and dermatologic diseases [9, 13, 19, 35–38]. The PROMIS-29 Profile is a recommended, easy-to-use tool for the measurement of a wide range of patient-centered outcomes in clinical trials [19, 38]. Use of generic PRO measures, such as the PROMIS Profile, HAQ-DI, and SF-36, allows for performance of comparative analyses measuring the effect of various diseases on the same health outcomes [3]. Although the PROMIS Profile is not specific to PsA, the components of this instrument are established as important PROs in comprehensive PsA disease management, and data suggest that PROMIS Profile components may be more sensitive than traditional PRO measures in detecting clinical improvement [5, 19, 39, 40]. However, it is possible that the observed treatment effects in DISCOVER-1 may have been different using a disease-specific PRO measure, such as the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire [41–43].
A limitation of this analysis is that the full PROMIS-29 Profile is not yet validated in patients with PsA, and clinically meaningful change thresholds (MCTs) have not been well established in patients with PsA. However, validation and establishment of MCTs of PROMIS Profile domains are areas of active research, and studies published to date support the use of PROMIS measures in patients with PsA [38–40, 44]. For example, it was recently established that a PROMIS-29 Profile physical function threshold score of ≥ 41.3 is equivalent to a HAQ-DI score of ≤ 0.5 when assessing minimal disease activity (MDA) physical function criteria in patients with PsA [39]. Furthermore, PROMIS-29 scores have been shown to be correlated with SF-36 and Veterans RAND 36 (VR-36; a modification of the SF-36) [45] scores and may have the potential to better measure health-related quality-of-life outcomes because of differences in scoring formulas [46, 47]. The MCS and PCS of the SF-36 and VR-36 are designed to be orthogonal (not correlated), while the mental and physical health scores of the PROMIS-29 are correlated with each other. Since impairments in physical health are known to affect mental well-being for many patients, it follows that the PROMIS-29 may more accurately reflect how most people experience the overall life impact of disease [46, 47]. This difference between the SF-36 and the PROMIS-29 may help explain the observation that in the DISCOVER-1 study, improvements in the SF-36 MCS did not reach statistical significance for guselkumab versus placebo at Week 24 [21], but improvements in PROMIS-29 T-scores for depression were nominally significant.
Another limitation of this analysis is that the sample size for DISCOVER-1 was determined to ensure statistical power for the primary endpoint of American College of Rheumatology (ACR) 20 response at Week 24, not for changes in PROMIS-29 scores from baseline to Week 24. Additionally, the standardized PROMIS-29 T-scores (mean = 50 for all domains) are based on normalized values in the general US population, whereas DISCOVER-1 is a global study that included patients from 13 countries. It is possible that the population norms across these 13 countries may differ from US norms. However, several studies have found that demographic factors, including age, gender, and language, have no or negligible effects on PROMIS T-scores, and that PROMIS item banks can be universally applied across diverse patient populations [48–52].
Conclusions
In patients with active PsA, PROMIS-29 Profile results indicate that treatment with guselkumab was associated with clinically meaningful improvements in health-related quality of life, including reductions in pain and fatigue, improvements in physical function, and increases in satisfaction with social participation. In the DISCOVER-1 study, these improvements in health-related quality-of-life domains were maintained through 1 year of treatment with guselkumab.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support was provided by Cherie Koch, PhD, of Janssen Scientific Affairs, LLC, under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2015;163:461-4).
Declarations
Funding
This study was funded by Janssen Research & Development, LLC.
Conflict of interest
Ana-Maria Orbai has received grant/research support from AbbVie, Amgen, Eli Lilly and Company, Celgene, Novartis, Janssen, and Horizon; has received consulting fees from Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, and is also a private consultant or advisor for Sun Pharmaceutical Industries, Inc, not in her capacity as a Johns Hopkins faculty member and was not compensated for this service. Laura C. Coates has received grant/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; speaker fees from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Medac, Novartis, Pfizer, and UCB; and consultant fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Laura C Coates is funded by a National Institute for Health Research (NIHR) Clinician Scientist award. The research was supported by the NIHR Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. We acknowledge the support of the NIHR Clinical Research Network. Atul Deodhar has received consulting fees for participation in Advisory Boards from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB; grant/research support from AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB; and speaker fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Philip S. Helliwell has received consulting fees from Eli Lilly and fees for educational services from AbbVie, Amgen, Novartis, and Janssen. Christopher T. Ritchlin has received grant/research support from UCB Pharma, AbbVie, and Amgen; consulting fees from UCB Pharma, Amgen, AbbVie, Lilly, Pfizer, Novartis, Gilead, and Janssen. Evan Leibowitz is an employee of Janssen Scientific Affairs, LLC, and owns stock in Johnson & Johnson, of which Janssen Scientific Affairs is a wholly owned subsidiary. Alexa P. Kollmeier, Elizabeth C. Hsia, Xie L. Xu, Shihong Sheng, Yan Liu, and Chenglong Han are employees of Janssen Research & Development, LLC, and own stock in Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary. Yusang Jiang is a consultant employed by Cytel, Inc. and funded by Janssen to provide statistical support.
Ethical approval
All procedures performed in this study involving human participants were conducted in accordance with the ethical standards of institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The protocol was approved by each site’s governing ethical body.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors have provided consent for publication of this article.
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Code availability
Not applicable.
Author contributions
Important intellectual contribution to conception and design, and/or acquisition of data, and/or analysis and interpretation of data provided by AMO, LCC, AD, PSH, CTR, EL, APK, ECH, XLX, SS, YJ, YL, and CH. Critical revisions of the manuscript for important intellectual content provided by AMO, LCC, AD, PSH, CTR, EL, APK, ECH, XLX, SS, YJ, YL, and CH. Final approval of the of the version to be published provided by AMO, LCC, AD, PSH, CTR, EL, APK, ECH, XLX, SS, YJ, YL, and CH. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved provided by AMO, LCC, AD, PSH, CTR, EL, APK, ECH, XLX, SS, YJ, YL, and CH.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 2.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta-Felquer ML, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 3.Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14:405–417. doi: 10.1080/1744666X.2018.1468252. [DOI] [PubMed] [Google Scholar]
- 4.Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum. 2017;47:351–360. doi: 10.1016/j.semarthrit.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis. 2017;76:673–680. doi: 10.1136/annrheumdis-2016-210242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coates LC, Orbai AM, Azevedo VF, Cappelleri JC, Steinberg K, Lippe R, et al. Results of a global, patient-based survey assessing the impact of psoriatic arthritis discussed in the context of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire. Health Qual Life Outcomes. 2020;18:173. doi: 10.1186/s12955-020-01422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogdie A, de Wit M, Callis Duffin K, Campbell W, Chau J, Coates LC, et al. Defining outcome measures for psoriatic arthritis: a report from the GRAPPA-OMERACT Working Group. J Rheumatol 2017;44:697–700. [DOI] [PMC free article] [PubMed]
- 8.HealthMeasures. https://www.healthmeasures.net/. Accessed 13 May 2022.
- 9.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. Initial adult health item banks and first wave testing of the Patient-Reported Outcomes Measurement Information System (PROMIS™) Network: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz P, Pedro S, Michaud K. Performance of the Patient-Reported Outcomes Measurement Information System 29-Item Profile in rheumatoid arthritis, osteoarthritis, fibromyalgia, and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017;69:1312–1321. doi: 10.1002/acr.23183. [DOI] [PubMed] [Google Scholar]
- 12.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the Patient-Reported Outcomes Measurement Information System (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis. 2015;74:104–107. doi: 10.1136/annrheumdis-2013-204053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett SJ, Orbai AM, Duncan T, DeLeon E, Ruffing V, Clegg-Smith K, et al. Reliability and validity of selected PROMIS measures in people with rheumatoid arthritis. PLoS ONE. 2015;10:e0138543. doi: 10.1371/journal.pone.0138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oude Voshaar MAH, ten Klooster PM, Glas CAW, Vonkeman HE, Taal E, Krishnan E, et al. Validity and measurement precision of the PROMIS physical function item bank and a content validity-driven 20-item short form in rheumatoid arthritis compared with traditional measures. Rheumatology (Oxford) 2015;54:2221–2229. doi: 10.1093/rheumatology/kev265. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett SJ, Gutierrez AK, Andersen KM, Bykerk VP, Curtis JR, Haque UJ, et al. Identifying minimal and meaningful change in PROMIS® for rheumatoid arthritis: use of multiple methods and perspectives. Arthritis Care Res (Hoboken) 2022;74:588–597. doi: 10.1002/acr.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacalao EJ, Greene GJ, Beaumont JL, Eisenstein A, Muftic A, Mandelin AM, et al. Standardizing and personalizing the treat to target (T2T) approach for rheumatoid arthritis using the Patient-Reported Outcomes Measurement Information System (PROMIS): baseline findings on patient-centered treatment priorities. Clin Rheumatol. 2017;36:1729–1736. doi: 10.1007/s10067-017-3731-5. [DOI] [PubMed] [Google Scholar]
- 17.Khanna D, Krishnan E, Dewitt EM, Khanna PP, Spiegel B, Hays RD. The future of measuring patient-reported outcomes in rheumatology: Patient-Reported Outcomes Measurement Information System (PROMIS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S486–S490. doi: 10.1002/acr.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orbai AM, Bingham CO., 3rd Patient reported outcomes in rheumatoid arthritis clinical trials. Curr Rheumatol Rep. 2015;17:28. doi: 10.1007/s11926-015-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyo RA, Ramsey K, Buckley DI, Michaels L, Kobus A, Eckstrom E, et al. Performance of a Patient Reported Outcomes Measurement Information System (PROMIS) Short Form in older adults with chronic musculoskeletal pain. Pain Med. 2016;17:314–324. doi: 10.1093/pm/pnv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TREMFYA® (guselkumab) injection for subcutaneous use. Horsham, PA: Janssen Biotech, Inc., July 2020 (package insert).
- 21.Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–1125. doi: 10.1016/S0140-6736(20)30265-8. [DOI] [PubMed] [Google Scholar]
- 22.Ritchlin CT, Helliwell PS, Boehncke WH, Soriano ER, Hsia EC, Kollmeier AP, et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open. 2021 doi: 10.1136/rmdopen-2020-001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 26 Jan 2022. [DOI] [PMC free article] [PubMed]
- 24.Bingham CO, 3rd, Butanis AL, Orbai AM, Jones M, Ruffing V, Lyddiatt A, et al. Patients and clinicians define symptom levels and meaningful change for PROMIS pain interference and fatigue in RA using bookmarking. Rheumatology (Oxford) 2021;60:4306–4314. doi: 10.1093/rheumatology/keab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and minimally important differences for 4 Patient-Reported Outcomes Measurement Information System Short Forms: physical function, pain interference, depression, and anxiety in knee osteoarthritis. J Pain. 2017;18:1096–1110. doi: 10.1016/j.jpain.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CX, Kroenke K, Stump TE, Kean J, Carpenter JS, Krebs EE, et al. Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain. 2018;159:775–782. doi: 10.1097/j.pain.0000000000001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. J Pain Res. 2016;9:251–255. doi: 10.2147/JPR.S93391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53:459–468. doi: 10.1016/S0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 29.Oak SR, Strnad GJ, Bena J, Farrow LD, Parker RD, Jones MH, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4:2325967116674714. doi: 10.1177/2325967116674714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman P, Mease PJ, Helliwell PS, Deodhar A, Gossec L, Kavanaugh A, et al. Guselkumab demonstrated an independent treatment effect in reducing fatigue after adjustment for clinical response-results from two phase 3 clinical trials of 1120 patients with active psoriatic arthritis. Arthritis Res Ther. 2021;23:190. doi: 10.1186/s13075-021-02554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnes IB, Rahman P, Gottlieb AB, Hsia EC, Kollmeier AP, Chakravarty SD, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73:604–616. doi: 10.1002/art.41553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz P, Kannowski CL, Sun L, Michaud K. Estimation of minimally important differences and patient acceptable symptom state scores for the Patient-Reported Outcomes Measurement Information System Pain Interference Short Form in rheumatoid arthritis. ACR Open Rheumatol. 2020;2:320–329. doi: 10.1002/acr2.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz P, Pedro S, Alemao E, Yazdany J, Dall'Era M, Trupin L, et al. Estimates of responsiveness, minimally important differences, and patient acceptable symptom state in five Patient-Reported Outcomes Measurement Information System Short Forms in systemic lupus erythematosus. ACR Open Rheumatol. 2020;2:53–60. doi: 10.1002/acr2.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaumont JL, Davis ES, Curtis JR, Cella D, Yun H. Meaningful change thresholds for Patient-Reported Outcomes Measurement Information System (PROMIS) fatigue and pain interference scores in patients with rheumatoid arthritis. J Rheumatol. 2021;48:1239–1242. doi: 10.3899/jrheum.200990. [DOI] [PubMed] [Google Scholar]
- 35.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63:1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook KF, Jensen SE, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. doi: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esaa F, Prezzano J, Pentland A, Wolf JR. The utility of PROMIS domain measures in dermatologic care. Arch Dermatol Res. 2021;313:17–24. doi: 10.1007/s00403-020-02074-1. [DOI] [PubMed] [Google Scholar]
- 38.Wan MT, Walsh JA, Craig ET, Husni ME, Scher JU, Reddy SM, et al. A comparison of physical function instruments in psoriatic arthritis: HAQ-DI vs MDHAQ vs PROMIS10 global physical health. Rheumatology (Oxford) 2021;60:2307–2316. doi: 10.1093/rheumatology/keaa591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chew E, Perin J, Grader-Beck T, Orbai AM. Measurement of minimal disease activity in psoriatic arthritis using PROMIS-Physical Function or the Health Assessment Questionnaire-Disability Index. Arthritis Care Res (Hoboken) 2022;74:151–160. doi: 10.1002/acr.24433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowell WB, Gavigan K, Kannowski CL, Cai Z, Hunter T, Venkatachalam S, et al. Which patient-reported outcomes do rheumatology patients find important to track digitally? A real-world longitudinal study in ArthritisPower. Arthritis Res Ther. 2021;23:53. doi: 10.1186/s13075-021-02430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Højgaard P, Klokker L, Orbai AM, Holmsted K, Bartels EM, Leung YY, et al. A systematic review of measurement properties of patient reported outcome measures in psoriatic arthritis: A GRAPPA-OMERACT initiative. Semin Arthritis Rheum. 2018;47:654–665. doi: 10.1016/j.semarthrit.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Cañete JD, Nolla JM, Queiro R, Rodríguez MJ, Ruiz M, Lizán L. Expert consensus on a set of outcomes to assess the effectiveness of biologic treatment in psoriatic arthritis: the MERECES study. J Rheumatol. 2020;47:1637–1643. doi: 10.3899/jrheum.191056. [DOI] [PubMed] [Google Scholar]
- 43.Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: Elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73:1012–1019. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 44.Leung YY, Orbai AM, Ogdie A, Hojgaard P, Holland R, Goel N, et al. Appraisal of candidate instruments for assessment of the physical function domain in patients with psoriatic arthritis. J Rheumatol. 2021;48:58–66. doi: 10.3899/jrheum.191119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, et al. Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. J Ambul Care Manage. 2004;27:263–280. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Rose AJ, Bayliss E, Huang W, Baseman L, Butcher E, García RE, et al. Evaluating the PROMIS-29 v2.0 for use among older adults with multiple chronic conditions. Qual Life Res. 2018;27:2935–2944. doi: 10.1007/s11136-018-1958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS®-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27:1885–1891. doi: 10.1007/s11136-018-1842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crins MH, Roorda LD, Smits N, de Vet HC, Westhovens R, Cella D, et al. Calibration and validation of the Dutch-Flemish PROMIS Pain Interference Item Bank in patients with chronic pain. PLoS ONE. 2015;10:e0134094. doi: 10.1371/journal.pone.0134094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crins MHP, Terwee CB, Ogreden O, Schuller W, Dekker P, Flens G, et al. Differential item functioning of the PROMIS physical function, pain interference, and pain behavior item banks across patients with different musculoskeletal disorders and persons from the general population. Qual Life Res. 2019;28:1231–1243. doi: 10.1007/s11136-018-2087-x. [DOI] [PubMed] [Google Scholar]
- 50.Oude Voshaar MA, ten Klooster PM, Glas CA, Vonkeman HE, Taal E, Krishnan E, et al. Calibration of the PROMIS physical function item bank in Dutch patients with rheumatoid arthritis. PLoS ONE. 2014;9:e92367. doi: 10.1371/journal.pone.0092367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paz SH, Spritzer KL, Reise SP, Hays RD. Differential item functioning of the patient-reported outcomes information system (PROMIS®) pain interference item bank by language (Spanish versus English) Qual Life Res. 2017;26:1451–1462. doi: 10.1007/s11136-017-1499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rawang P, Janwantanakul P, Correia H, Jensen MP, Kanlayanaphotporn R. Cross-cultural adaptation, reliability, and construct validity of the Thai version of the Patient-Reported Outcomes Measurement Information System-29 in individuals with chronic low back pain. Qual Life Res. 2020;29:793–803. doi: 10.1007/s11136-019-02363-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.