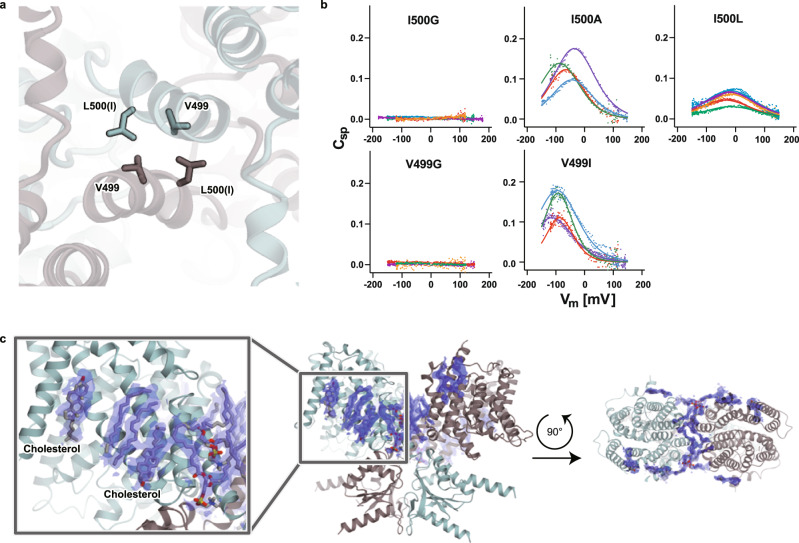
Fig. 6. Dimerization of PresTS.
a Hydrophobic interactions are mediated by V499 and L500 (I500 in wild-type) at the C-termini of the TM14 helices. b Cell membrane electric capacitance measurements in HEK293T cells expressing I500G-, I500A-, I500L-, V499G-, and V499I-HgPres. Five examples in different colors are shown for each panel. Solid lines (for V499I-, I500A-, and I500L-HgPres) indicate two-state Boltzmann fittings. The α, Vpk, and charge density values (mean ± S.D.) were as follows: [0.027 ± 0.008 mV−1, −104 ± 21 mV, 23 ± 14 fC/pF] for V499I (n = 6); [0.025 ± 0.006 mV−1, −58 ± 36 mV, 20 ± 9 fC/pF] for I500A (n = 8); and [0.017 ± 0.001 mV−1, −22 ± 8 mV, and 13 ± 4 fC/pF] for I500L (n = 5). Source data are provided as a Source Data file. c Lipids found on the PresTS structure. Phospholipids and cholesterols are shown as gray stick models with cryo-EM densities (blue). A close-up view of lipids bound to PresTS (left panel). A lateral view (center panel) and an extracellular view (right panel) of lipids found on PresTS.