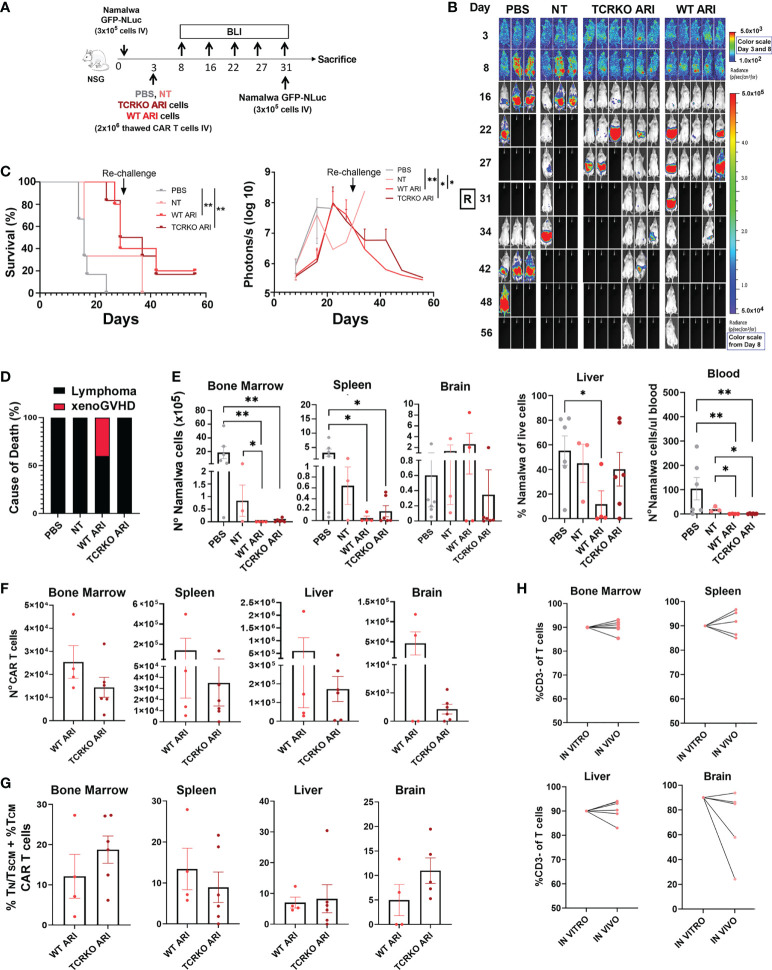
Figure 6.
In vivo anti-tumor activity and phenotype of TCRKO ARI-0001 cells. (A) Schematic overview of the in vivo experimental design. (B) Representative BLI images of tumor burden at different days after Namalwa inoculation. A rechallange with new Namalwa cells was performed with alive mice at day 31. Color scale bar is shown for Day 3 and Day 8-56. Radiance scale is shown in each bar. (C) Left: Overall survival. Mice with > 20% body weight loss or high bioluminescence signal (>5x107 Photons/sec) were sacrified (N=3 mice for untreated (PBS) and non-transduced (NT), N=5 mice for WT ARI-0001 and N=6 mice for TCRKO ARI-0001. CAR T cells were obtained from one healthy donor. Right: radiance quantitative trace of each group of mice represented as photons per second at the different days. (D) Incidence (%) of lymphoma- or xenoGVHD- related death in the different mouse groups. (E) Number of Namalwa cells in bone marrow, spleen, liver, brain and blood in NSG mice left untreated (PBS) or treated with NT, ARI-0001 CAR-T cells (WT ARI) and TCR-deficient ARI-0001 cells (TCRKO ARI) at the end point. (F) Number of CAR-T cells in bone marrow, spleen, liver and brain in NSG mice treated with WT ARI-0001 or TCRKO ARI-0001 cells at the point. G) Percentage of TN/TSCM +TCM population in hCD2+CAR+ cells analyzed in different mice organs after sacrifice. Only when CAR+ population was >1%, data was included. (H) Percentage of hCD3- cells of live cells prior inoculation (in vitro) and %hCD3- cells of hCD2+ cells in liver, bone marrow, spleen and brain after sacrifice (in vivo). Statistics are based on log rank test (C), unpaired, one-tailed Student´s t-test (D), *p<0.05, **p<0.01. Graphs show mean ± SEM.