Scheme 1. Synthesis of Derivatives 14a–i.
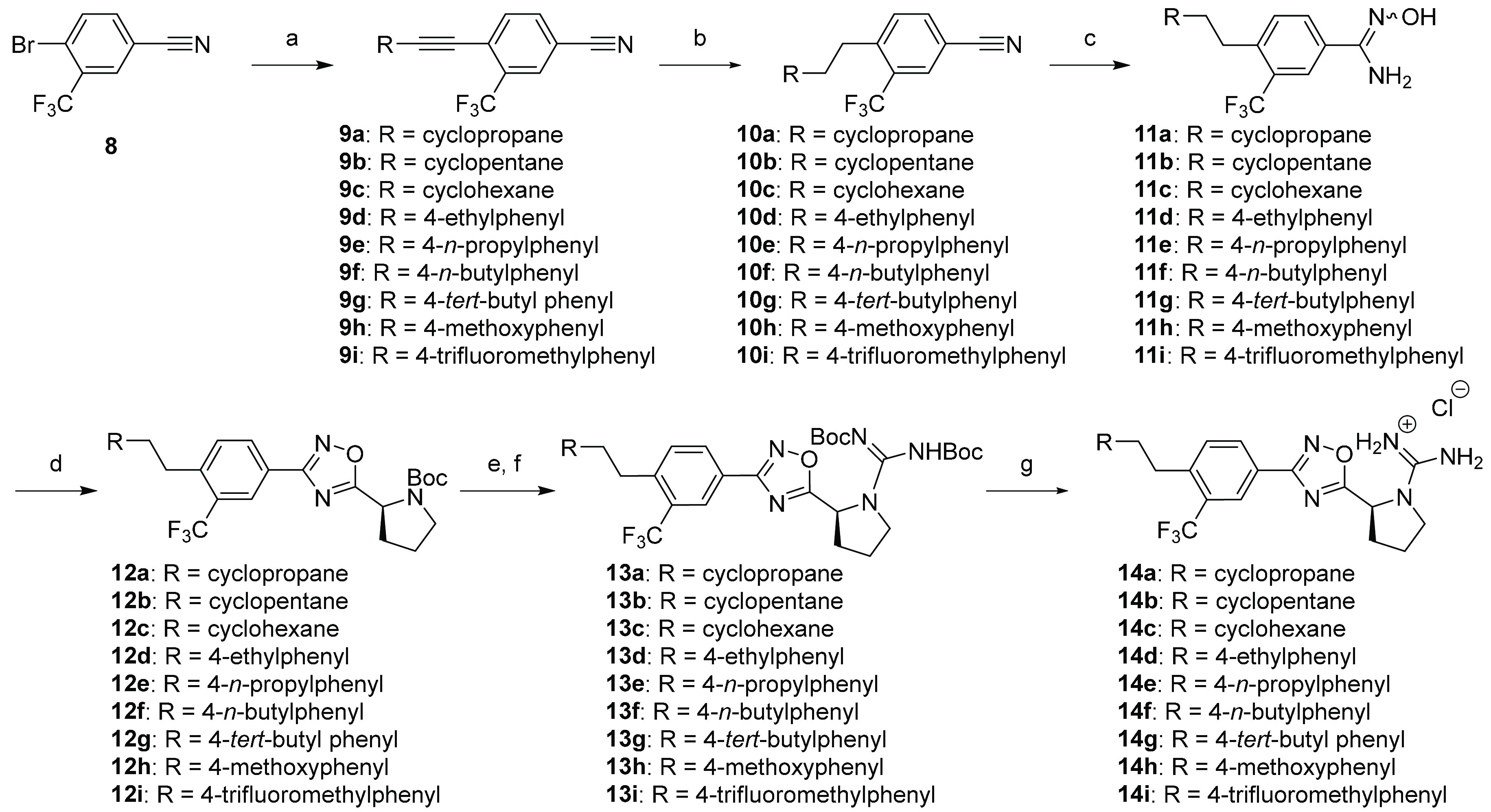
Reaction conditions: (a) Alkyne, Pd(PPh3)2Cl2, CuI, TEA, DMF, 80 °C, 4–12 h; (b) Lindlar’s catalyst, H2, EtOAc, 1–20 h; c) NH2OH·HCl, TEA, EtOH, 80 °C, 6 h; (d) Boc-l-proline, DIEA, HCTU, DMF, 110 °C, 18 h; (e) HCl(g)/MeOH, rt, 4 h; (f) N,N′-di-Boc-1H-pyrazole-1-carboxamidine, DIEA, MeCN, 55 °C, 2–4 h, microwave; (g) 4M HCl/Dioxane, rt, 3 h.
