Scheme 2. Synthesis of Ether Tail Derivatives 23a–i.
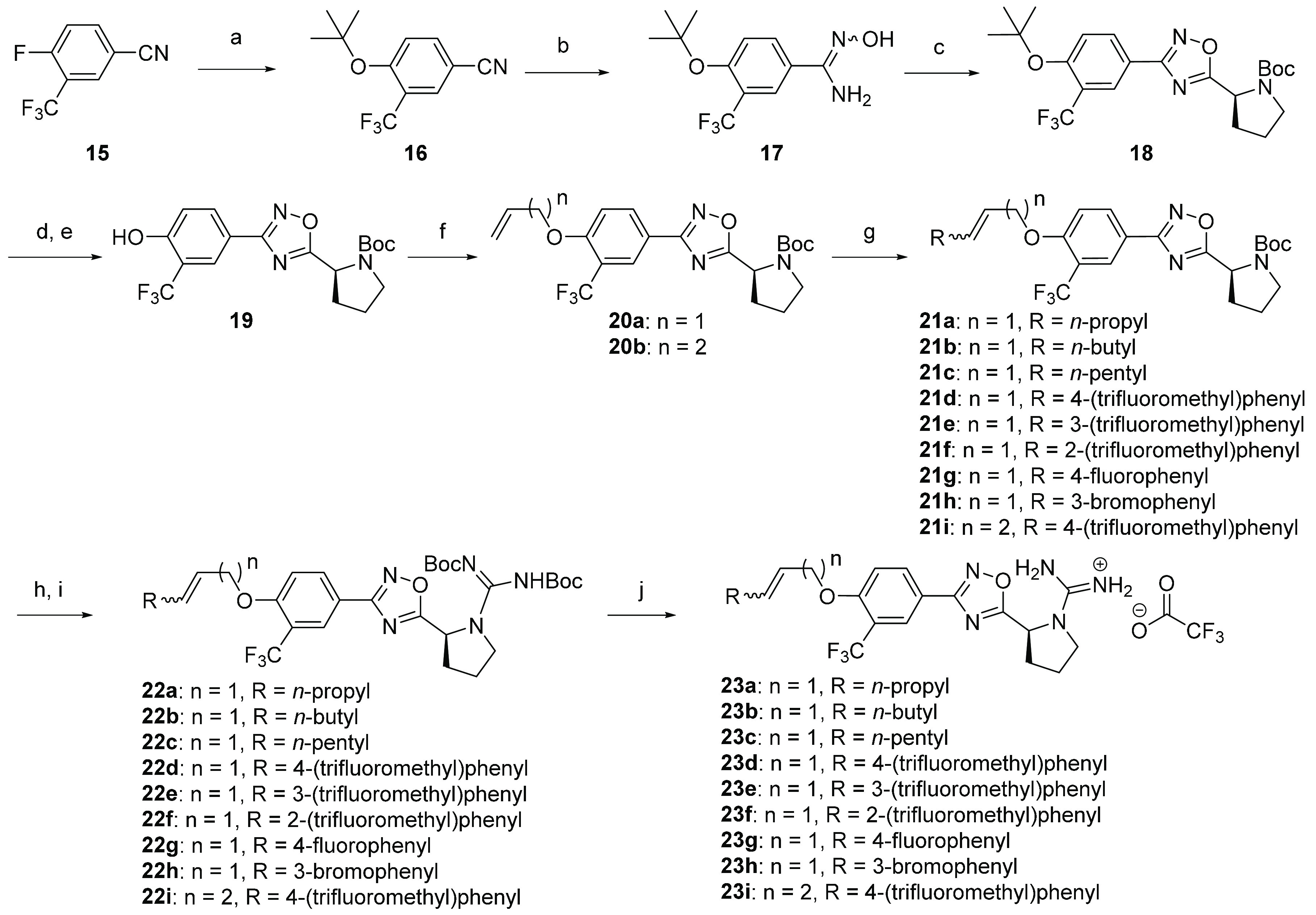
Reaction conditions: (a) KOtBu, THF, 60 °C, 4 h; (b) TEA, NH2OH·HCl, TEA, EtOH, 80 °C, 6 h; (c) Boc-l-proline, DIEA, HCTU, DMF, 110 °C, 18 h; (d) TFA, DCM, 3–6 h, 0 °C to rt; (e) (Boc)2O, TEA, THF, rt, 2–5 h; (f) alkyl bromide, K2CO3, acetone, 90 °C, 2 h, microwave; (g) Grubbs II catalyst, terminal alkenes, DCM, 70 °C, 2–4 h, microwave; (h) TFA, DCM, 0 °C to rt, 3–6 h; (i) N,N′-Di-Boc-1H-pyrazole-1-carboxamidine, DIEA, MeCN, 55 °C, 2–6 h, microwave; (j) TFA, DCM, rt, 4 h.
