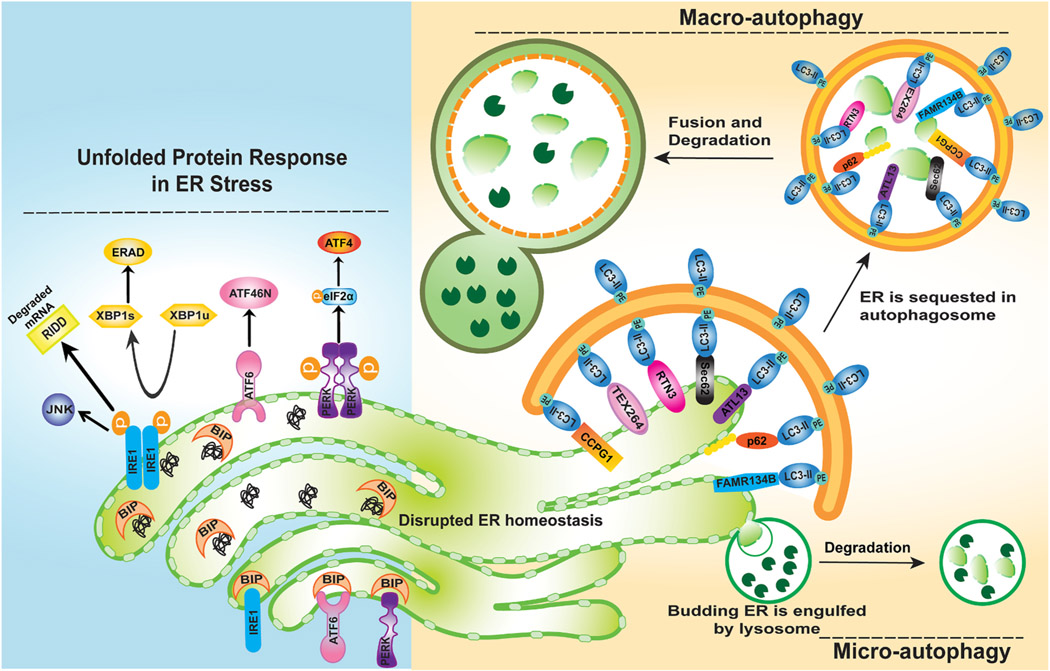
Fig. 4. ER-phagy during ER stress.
During ER stress, the stress sensors IRE1, ATF6, and PERK which are originally bound to BIP, are activated by dissociating from BIP. Activated IRE1 dimers trigger JNK activation and induce processing of un-spliced XBP1 (XBP1u) mRNA into its spliced form (XBP1s), which regulates protein production involved in the ERAD pathway. IRE1 also activates the RIDD pathway to degrade mRNAs localize to the ER membranes. Activated PERK phosphorylates eIF2α, which inhibits global translation and selectively increases mRNA translation of some stress-related proteins such as ATF4. Activated ATF6 is processed into active N-terminus cytosolic fragment (ATF6N) which translocates to the nucleus and regulates the expression of ER-stress related genes. In the liver, the exposure of hepatocytes to xenobiotics can result in proliferation of smooth ER to produce cytochrome P-450 (CYPs) enzymes that mediate xenobiotic metabolization. ER-phagy plays an important role in restoring ER homeostasis by removing damaged and excessive ER. Both micro-autophagy and macro-autophagy can be involved in removing ER. In micro-autophagy lysosome membrane invagination directly engulfs ER fragments, whereas in macro-autophagy, the ER fragments are first packed into autophagosomes through selective autophagy receptors before fusing with lysosomes for degradation. The selective ER-phagy receptors include but are not limited to TEX264, RTN3L, CCPG1, FAM134B, ATL3, p62 and SEC62. These receptor proteins are either ER resident proteins or can interact with ER membrane proteins, but all receptor proteins contain a LIR domain to directly interact with LC3 to recruit autophagosomes to the ER.