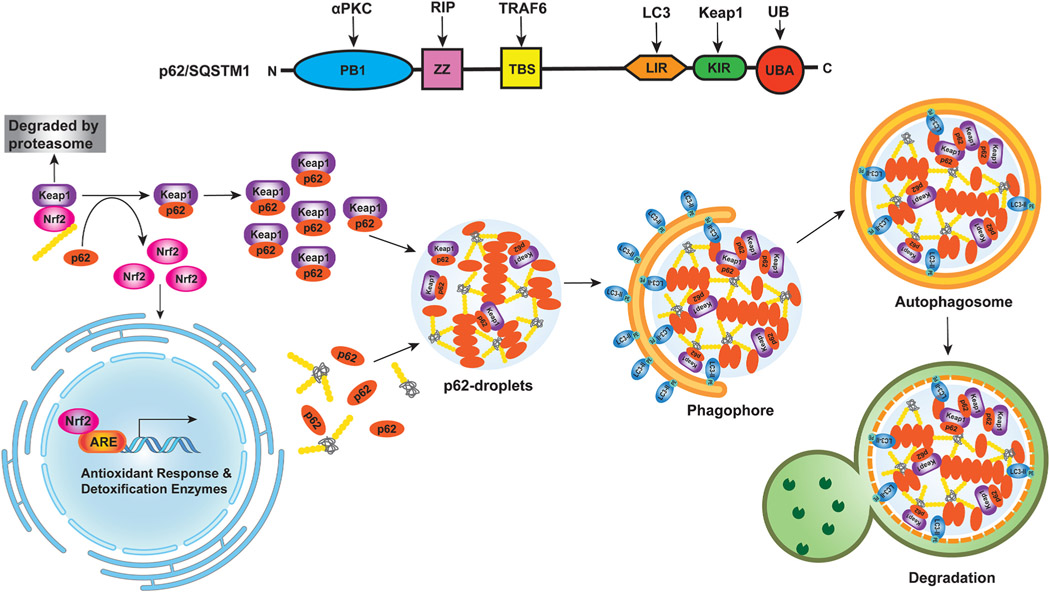
Fig. 5. p62-Nrf2 activation in liver cancer.
(1) Schematic illustration of p62 domain structure. Phox/Bemp1 (PB1) domain is involved in the binding of p62 to atypical PKC (aPKC) or ERK1. The ZZ zinc finger region and the TRAF6-binding domain, TBS, interact with RIP1 and TRAF6, respectively, to activate the p62-mediated nuclear factor-κB (NF-κB) pathway. LIR domain of p62 binds to LC3 protein to trigger the selective-autophagy pathway. KIR domain next to LIR binds to Keap1 and transports Keap1 into the autophagosome for degradation, which leads to the activation of the non-canonical Keap1-Nrf2 pathway. The ubiquitin-associated (UBA) domain on the C terminus binds to ubiquitinated proteins. Both the PB1 and UBA domains play an important role in p62-mediated aggregate formation. (2) Crosstalk between Nrf2 and p62 phase separation in selective autophagy. p62 regulates the Keap1-Nrf2 pathway through the KIR domain. In canonical Keap1-Nrf2 pathway, Keap1 mediates Nrf2 ubiquitination and leads to Nrf2 degradation via the proteasome. In non-canonical Keap1-Nrf2 pathway, p62 competitively binds to Keap1 leading to the stabilization of Nrf2 that can then translocate into the nucleus and activate the expression of antioxidant response-related detoxification enzymes, which protects against drug-induced hepatotoxicity and cancer cell survival. p62 is a multivalent protein and multivalent interactions between p62 and poly-ubiquitin or ubiquitinated misfolded proteins promotes p62 droplet formation, which can also recruit Keap1. The liquid-like properties of the p62 condensates are also thought to be crucial in the initiation of autophagosome formation for aggrephagy.