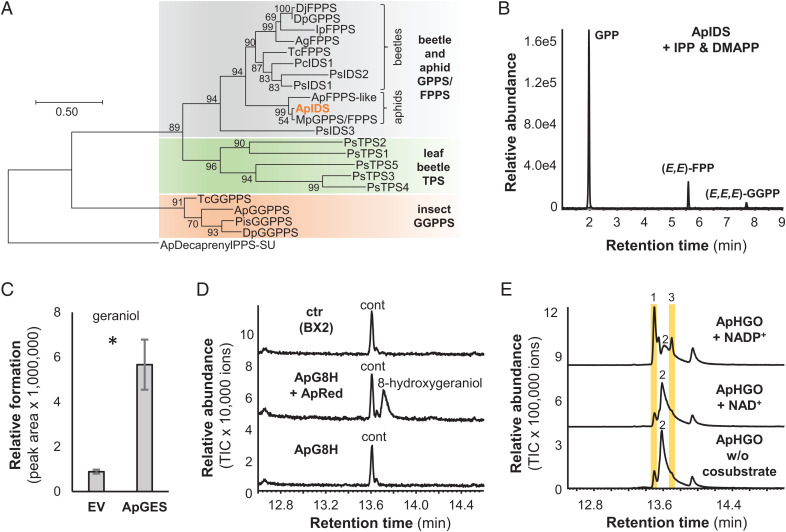
Fig. 2.
Biochemical characterization of ApIDS, ApGES, ApG8H, and ApHGO. (A) Cladogram analysis of characterized IDSs and TPSs from insects. The tree was inferred by using the maximum likelihood method based on the Jones-Thornton-Taylor (JTT) model. Bootstrap values (n = 1,000) are shown next to each node. The rooted tree is drawn to scale, with branch length measured in the number of amino acid substitutions per site. Putative decaprenyldiphosphate synthase subunit 2 from A. pisum was used as an outgroup. (B) Characterization of ApIDS. ApIDS lacking the N-terminal signal peptide was expressed as an N-terminal His-tag fusion protein in E. coli, purified, and incubated with the substrates IPP and DMAPP in the presence of 1 mM CoCl2. Enzyme products GPP, (E,E)-FPP, and (E,E,E)-GGPP were analyzed using liquid chromatography–tandem mass spectrometry. (C) Characterization of ApGES. The phosphatase ApGES was expressed in S. cerevisiae and microsomes harboring the recombinant protein were incubated with GPP. Geraniol was extracted with hexane and analyzed using GC-MS. Mean peak area ± SE are shown (n = 3 independent expression constructs). The amount of geraniol formed was significantly higher in ApGES samples than in the negative controls (empty vector [EV]) (t = 5.219; df = 2.026; P = 0.034). (D) Characterization of ApG8H. S. cerevisiae microsomes containing either ApG8H, ApG8H in combination with the P450 reductase ApRed, or the maize P450 BX2 as negative control were assayed with geraniol as substrate and NADPH as cosubstrate. Reaction products were analyzed using GC-MS. cont, di-tert-butylphenol (contamination). (E) Characterization of ApHGO. ApHGO was expressed as N-terminal His-tag fusion protein in E. coli, purified, and incubated with 8-hydroxygeraniol either in the absence or presence of NAD(P). Enzyme products were extracted from the assays and analyzed using GC-MS. 1,8-oxogeraniol (partially oxidized product); 2,8-hydroxygeraniol (starting material); 3,8-oxogeranial (fully oxidized product). Note: The tailing of the peaks is due to the polar nature of the aldehydes and the alcohol.