Fig. 3.
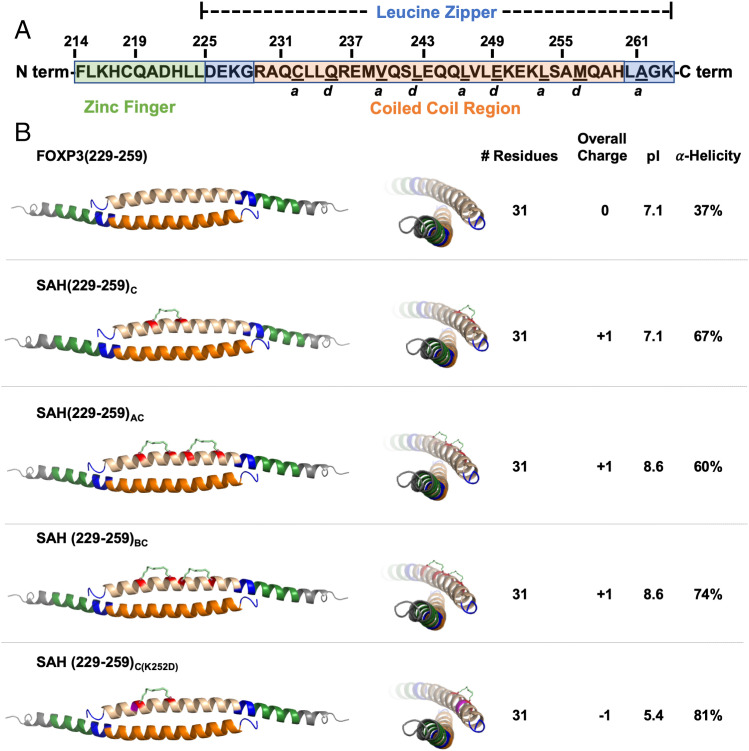
Double stapling of peptides modeled after SAH(229–259)C. (A) Human FOXP3 sequence. Residues F214–K263 represent the entirety of a helix constituting the zinc finger (F214–L224; shown in green) and leucine zipper (D225–K263; shown in blue) regions. Within the leucine zipper, the coiled-coil (R229–H259; shown in orange) represents an area that is highly conserved between human and murine FOXP3. Underlined residues indicate a and d core residues, which bind each other on an identical antiparallel helix and are key contact points for homodimerization. The homodimerized FOXP3 helix is labeled according to the color codes in the sequence above. (B) Design strategy for SAHs modeled after SAH(229–259)C. SAHs share sequence and length, differing in position and number of staples. These changes influence overall charge, pI, and alpha-helicity. Each three-dimensional structure shown is based on the crystal structure [PDB: 4I1L (26)]; the upper helix represents each SAH (shown in tan) and hydrocarbon staple (insertion sites shown in red).