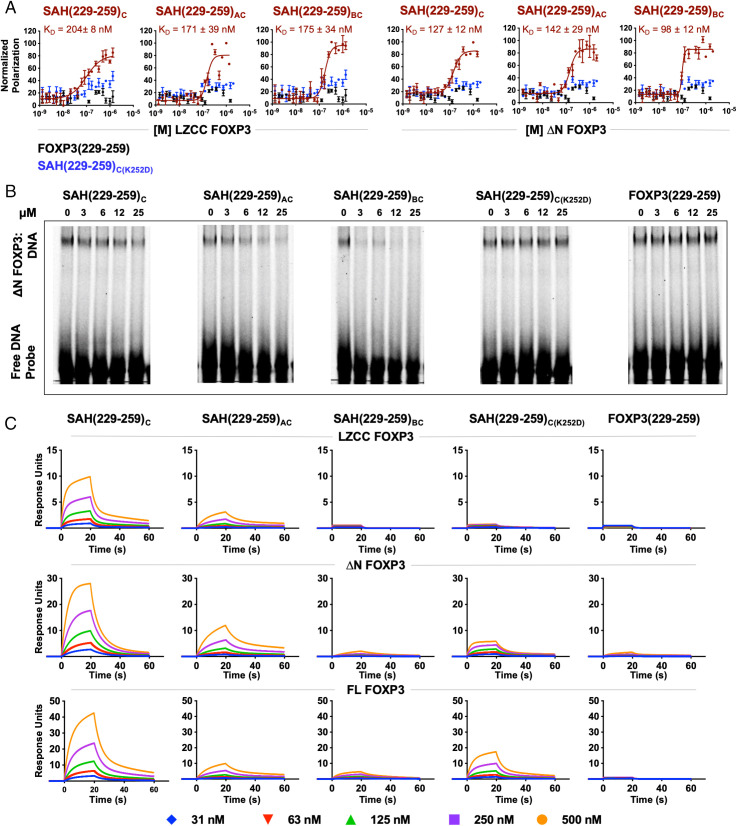
Fig. 4.
Biochemical characterization of SAH(229–259)C constructs. (A) FPA binding curves of SAHs (maroon), their point mutant control (blue), and native unstapled peptide (black) bound to either LZCC FOXP3 or ΔN FOXP3. SAHs bind to protein (KD) in the nanomolar range. Point mutant control and native unstapled peptides do not bind either FOXP3 construct. (B) Reduction in ΔN FOXP3 binding to consensus DNA was observed following treatment with SAH(229–259)C derivatives, but not native peptide or point mutant control, as measured by EMSAs. (C) Surface plasmon resonance (SPR) of SAHs shows differences in binding kinetics between single- and double-stapled peptides against LZCC FOXP3, ΔN FOXP3, and full-length FOXP3.