Abstract
WangShiBoChiWan (WSBCW) is a commonly used Chinese herbal medicine for the treatment of functional gastrointestinal disorders. However, its preclinical efficacy and the mechanisms of action have not been adequately studied. The goals of this study were to evaluate the effects of WSBCW on gastrointestinal health and modulation of related biomarkers. Female C57BL mice were randomly assigned into one of the experimental groups consisting of the control, drug controls, and WSBCW at 40, 120, and 360 mg/kg BW. Whole gut transit, small intestinal motility, and intestinal barrier permeability were determined. The castor oil-induced diarrhea mouse model was used to determine the effect of WSBCW on the diarrhea type of irritable bowel syndrome (IBSD). WSBCW increased whole gut transit and intestinal motility, improved intestinal permeability in healthy animals and alleviated diarrhea symptoms in IBS-D mice. WSBCW upregulated intestinal junction proteins, increased the abundance of Bifidobacterium genus, Desulfovibrio genus and inhibited Bacteroides fragillis group in the gut microbiota, increased intestinal villi lengths, and decreased blood levels of inflammatory cytokines. Our study provided preclinical evidence to verify the effectiveness of WSBCW in gastrointestinal health and elucidate mechanistic insights. The results warrant further investigations to evaluate the therapeutic efficacy of WSBCW on gastrointestinal disorders, such as IBS and IBD.
Keywords: Gastrointestinal, Motility, Permeability, Inflammation, Junction, Microbiota
1. Introduction
Gut health has become an essential objective in medicine through the past decades [1]. Irritable bowel syndrome (IBS) [2] is one of the most common functional gastrointestinal disorders (FGID). Although symptoms of FGID, such as constipation, diarrhea and abdominal pain, mostly indicate non-lethal diseases, they profoundly impair the quality of life. Epidemiological investigations have found high prevalence of FGID worldwide across different ages: infants/toddlers (27–41%) [3], children (20–29%) [3], adolescent (10–29%) [4], and adults (10–36%) [5]. Previous research has also shown that the ratio of prevalence between females and males can up to 3:1 [5]. Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC) that are characterized by chronic inflammation of gastrointestinal tract, severely affects life quality of adults worldwide at their productive ages.
Conventional treatments to mitigate symptoms of IBS, such as dietary manipulation, antispasmodics, antidiarrheals, laxatives and antidepressants, either lack adequate scientific evidence in proving their effectiveness or show side effects such as abdominal pain, dry mouth, nausea, vomiting, or dizziness. Gut microbiota correlating to FGID has been widely studied during the past years. Probiotics, such as Lactobacillus and Bifidobacterium, have beneficial properties for the host’s gut health and were reported to be potential for IBS patients [6]. However, many controlled trials of probiotics in IBS patients ended up with moderately positive results and unconvincing evidence [7]. Thus, current treatment regimens for IBS are unsatisfactory, and new strategies are urgently required. For IBD treatment, anti-bacteria agents, corticosteroids, and immunomodulators are commonly used in the purpose of eliminating inflammation [8]. Additionally, surgery is required in serve IBD patients to remove the damaged portions of gastrointestinal tract. Effective actions on prevention of IBD are of great benefits before it turned into server phases.
Herbal medicine has been commonly used for the prevention and treatment of gastrointestinal disorders, including IBS and IBD. WangShiBoChiWan (WSBCW) is a traditional Chinese medicine that was originally formulated in the Ching dynasty and its recipe includes Radix et Rhizoma Rhei, Rhizoma coptidis, Rhizoma Arisaematis, Buibus Fritillariae cirrhosae, and Fructus Crotonis. WSBCW has been reported to regulate gastroenterological functions and treat gastrointestinal disorders [9], such as diarrhea, functional constipation, functional cacochylia, IBS and chronic gastritis, in infants, children and adults since it was produced centuries ago. However, the literatures are mostly presented in Chinese and are not easily accessible. Moreover, the preclinical evidence on its efficacy has been inadequate and its underlying mechanism of action remains elusive. Therefore, it is essential to conduct preclinical investigations to evaluate its efficacy in modulating gastrointestinal functions and in elucidating possible mechanisms of action so that the supporting scientific evidence can be generated. Thus, this study aimed to evaluate the effects of WSBCW on improving gastrointestinal functions and alleviating symptoms in IBS, and to determine associated biomarkers.
2. Materials and methods
2.1. Chemicals and reagents
WSBCW is a prescribed herbal medicine approved by the Chinese Food and Drug Administration and it is manufactured by Jinghua Pharmaceutical Group Co., Ltd (Nantong, China). Its quality is controlled by the fingerprint profiles of its chemical components. Loperamide (LPM), FITC-Dextran and Evans blue were purchased from Sigma-Aldrich (St. Louis, MO). Linaclotide (LNT) was purchased from MedChemExpress (Monmouth Junction, NJ).
2.2. Animals and treatments
Eight-week-old female C57BL/6 mice were purchased from Taconic (Germantown, NY). After one-week acclimation, mice were randomly assigned into one of the following six experimental groups (n = 8/group): control (CON, PBS), control of decreasing transit drug loperamide (5 mg/kg BW), control of increasing transit drug linaclotide (100 μg/kg BW), WSBCW-Low (40 mg/kg BW), WSBCW-Middle (120 mg/kg BW), and WSBCW-High (360 mg/kg BW). The WSBCW doses were designed based on human use. An adult (60 kg BW) consumes 300 mg x 2 daily, which is approximately equivalent to a daily dose of 120 mg/kg BW. Both lower and higher doses were also included for evaluation. The animals in all experimental groups consumed the AIN-93M diet throughout the experiment. The animal experiment was performed in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
2.3. Whole gut transit assay
Whole gut transit assay was conducted after mice were treated with the corresponding regimens via oral gavage for one week. In the morning of the experiment, mice were fasted for 3 h in individual cages without bedding. Mice in each group were then orally administrated with vehicle (PBS), loperamide, linaclotide, WSBCW-Low, WSBCW-Middle, or WSBCW-High. After one hour, mice were orally given 150 μL of pre-warmed (37 °C) Evans blue marker (5% Evans blue, dissolved in pre-warmed 0.9% sodium chloride (NaCl, w/v) supplemented with 1.5% methylcellulose). Mice were allowed free access of food and water one hour later, and were monitored for 5 h. The time from the end of gavage to the elution of first blue fecal pellet was recorded in minutes and constituted the whole gut transit time.
2.4. Small intestinal motility assay
Mice were pretreated with PBS (vehicle, loperamide, and linaclotide groups) or WSBCW at corresponding doses for two weeks. After overnight fasting, mice in each group were given corresponding treatment regimens as described above, followed by Evans blue marker. After 30 min, mice were sacrificed, and the intestine was removed. Small intestinal motility was determined by measuring the migration distance of Evans blue from the pylorus to the most distal point of movement.
2.5. Barrier permeability assay
Mice were pretreated with PBS (vehicle, loperamide, and linaclotide groups) or WSBCW at corresponding doses for two weeks. After overnight fasting, mice were given corresponding treatment regimens as described above, followed by 100 μL of FITC-Dextran (100 mg/mL). Ninety minutes after FITC-Dextran gavage, blood samples were collected for serum preparation. The serum from each mouse was transferred to a new tube and diluted appropriately, and the fluorescence (FL) intensity of serum was measured at wavelengths of 485 nm for excitation and 535 nm for emission. Mice were sacrificed, blood samples, intestinal tissues, and ceca were collected for measurements of circulating cytokines, histopathology/biomarkers, and microbiota, respectively.
2.6. Preventive and therapeutic effects of WSBCW on castor oil induced IBS-diarrhea (IBS-D) model
Eight-week-old female C57BL/6 mice were randomly assigned into one of six experimental groups (n = 8/group): negative control (vehicle), model control (castor oil), drug control (loperamide, 5 mg/kg BW), models with WSBCW treatment at 40, 120, or 360 mg/kg BW. After 1 week of acclimatization and in the morning of the experiment, mice were fasted for 3 h, transferred to individual empty plastic cages without bedding and were left to acclimatize to the cage for 1 h. For investigation of preventive effect of WSBCW, mice in each group were treated accordingly via oral gavage 30 min before 150 μL of castor oil gavage (except the NC group). For investigation of therapeutic effect of WSBCW, mice were treated accordingly right after 150 μL of castor oil gavage. The mice were allowed free access to food and water after gavage, and were monitored every 5 min for 4 h. The time from the end of gavage to the first watery stool elution was measured in minutes. For those mice that did not elute watery stool through the observation, the time was recorded as 4 h for data analysis.
2.7. Genomic DNA extraction and quantitative PCR for determination of gut microbiota
The microbial genomic DNA in cecum was extracted from 100 mg of each fecal sample using the Quick-DNATM Fecal/Soil Microbe Miniprep Kit (D6010, Zymo Research, USA) according to the manufacturer’s instructions. The total DNA samples were quantified and assessed purity using NanoDrop™ 2000 spectrophotometers (ThermoFisher Scientific, USA). The amount of total microbiota was estimated by using the universal primers, Uni331F and Uni797R, which amplified a conserved region of the 16 S rRNA for most microbiota. The following representative dominant/subdominant groups from four major phyla of gut microbiota were chosen: Atopobium cluster, Bifidobacterium genus, Bacteroides fragilis group, Clostridium coccoides group, Desulfovibrio genus, Lactobacillus genus, Clostridium leptum group, and Prevotella genus. The specific primers were selected and qPCR assays were performed following the protocols we described previously [10].
2.8. Quantitative real-time PCR for analysis of intestinal permeability related genes
RNA was extracted and purified from 50 mg of the proximal small intestine section using the TRIzol Reagent (ThermoFisher Scientific, USA) and Direct-zol™ RNA Miniprep Plus (ZYMO Research, USA) according to the manufacturer’s instructions. The total RNA samples were quantified and assessed purity using NanoDrop™ 2000 spectrophotometers (ThermoFisher Scientific, USA). RNA was reverse transcribed to cDNA using Superscript™ VILO™ MasterMix (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. The specific primers for intestinal permeability related genes were either designed using primer blast or selected from previous references and the sequences were listed in Table 1. qRT-PCR assays were performed following the protocols we described previously [10].
Table 1.
The primer sequences for quantitative real-time PCR analysis of tight junction and adherens junction genes.
| Forward | Reverse | |
|---|---|---|
| ZO-1 | GAGACAAGATGTCCGCCAGG | AAATCCAAACCCAGGAGCCC |
| ZO-2 | ATGGGAGCAGTACACCGTGA | TGACCACCCTGTCATTTTCTTG |
| ZO-3 | TCGGCATAGCTGTCTCTGGA | GTTGGCTGTTTTGGTGCAGG |
| Claudin-1 | GGCTTCTCTGGGATGGATCG | TTTGCGAAACGCAGGACATC |
| Claudin-2 | TGCGACACACAGCACAGGCATCAC | TCAGGAACCAGCGGCGAGTAGAA |
| Claudin-3 | GACCGTACCGTCACCACTAC | CAGCCTAGCAAGCAGACTGT |
| Claudin-4 | ACACGTTACTCCAGCGCTAC | CTCTCAATGGCCCCTCAGTC |
| Claudin-7 | TCCTGTACCTACCTGGTCCT | CGAGTTGGCCATTTCCTTGTC |
| Claudin-15 | TGGGCAACATGGATCTCTCC | AGTGATGTTGACGGCGTACC |
| cdh1 | TCCTTGTTCGGCTATGTGTC | GGCATGCACCTAAGAATCAG |
| jam1 | ACCCTCCCTCCTTTCCTTAC | CTAGGACTCTTGCCCAATCC |
| jam4 | GGACTCAGAGGCTCACTTCA | AGACTCAGCACCACCATTTG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
2.9. Histology analysis
The proximal small intestine (2 cm) was excised and fixed in 4% paraformaldehyde. Three sections of each intestine sample at random locations were sliced vertically on a slide and processed for H&E staining. Photos were taken at 4x magnificence with AmScope camera. Distances from bottom to top of the total villi in the slide were measured with AmScope software. Villi length for each individual mouse was averaged from lengths of all villi in three sections on one slide.
2.10. MILLIPLEX multiplex assay
Serum levels of cytokines (monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor (TNF)-α, interleukin (IL)-12 (p40), IL-12 (p70), IL-2, IL-6 and interferon (IFN)-γ) were determined with the MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel (MilliporeSigma; USA) and established protocols following the manufacturer’s instructions.
2.11. Statistical analysis
Data were expressed as mean ± standard error and analyzed by one-way analysis of variance (ANOVA) test, then multiple comparisons of the least-significant difference (equal variances assumed) or Dunnett’s T3-test (equal variances not assumed) were used to evaluate the difference of parametric samples among groups. Analyses of MILLIPLEX MAP data for cytokines considered data that were above the detection limits. Concentrations obtained below the sensitivity limit of detection of the method were recorded as 0 for statistical comparisons. If raw data or log-transformed data did not meet the statistical criteria for the assumption of normality showing equal variance, the nonparametric Kruskal-Wallis and Mann-Whitney test were used to determine statistical differences, and Bonferroni correction was used to correct p value. Statistical significance of the frequency of mice developed water stool after castor oil administration was determined by χ2 analysis. Differences were considered to be statistically significant at P < 0.05. All analyses were carried out using GraphPad Prism 8 Software.
3. Results
3.1. WSBCW enhanced whole gut transit and intestinal motility
In the whole gut transit assay, the elution time of first fecal containing Evans blue for each group was shown in Fig. 1A. Loperamide treated mice showed significantly delayed elution time than the control group, while linaclotide treated mice showed significantly shortened elution time. Mice treated with WSBCW at the low or the middle dose had significantly shortened elution times than the control group. However, WSBCW at the high dose did not show a significant difference compared with the control group.
Fig. 1.
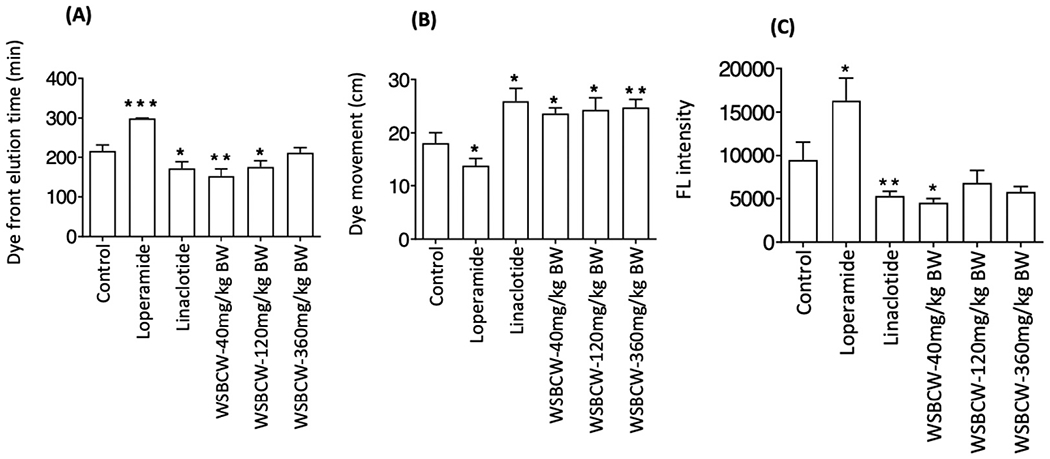
Effects of WSBCW on gastrointestinal health in normal situation. (A) Whole gut transit after one-week pretreatment, (B) small intestine motility of mice after two-week pretreatment, and (C) small intestinal permeability as measured by FL intensity of FITC-dextran permeated into the serum of mice. Within each panel, values with “*” are significantly different from that of the control; *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 1B showed the migrated distance of Evan blue in small intestine. Among all groups, linaclotide treatment showed the fastest movement of Evans blue in small intestine, and loperamide treatment showed the slowest movement, the results consistent with the functions of these control drugs. WSBCW at the experimental doses significantly enhanced transit of Evans blue, to the levels similar to that of linaclotide treatment. The results from intestine motility assay agreed with that from the whole gut transit assay, suggesting that WSBCW improve whole gut transit through improving small intestine motility. WSBCW treatments for two weeks did not significantly alter food intake or body weight (data not shown), indicating minimal adverse effect.
3.2. WSBCW improved intestinal permeability and upregulated the expression of tight junctions and adherens junctions
Fig. 1C showed the FL intensity of serum in each group of mice under indicated treatment. Loperamide treated group showed significantly increased FL intensity of serum compared with the control group, indicating higher intestinal permeability. Linaclotide- and the low dose WSBCW- treated groups showed significantly decreased FL intensity of serum compared with the control group. While WSBCW-Middle and WSBCW-High treatments also showed lower intestinal permeability than the control group, the differences were not statistically significance.
Since the WSBCW at the low dose showed more significant effect on gastrointestinal functions (whole gut transit, small intestinal motility, gut barrier permeability) than that at the middle or high dose, the samples from the control group, WSBCW-Low, and WSBCW-High groups were selected to cover a wide range of doses to further investigate the effect of WSBCW on alteration of molecular biomarkers. The expression of intestinal tight junctions and adherens junctions related genes were evaluated using the RT-PCR method. Results were shown in Fig. 2. Compared with the control group, the WSBCW-Low group showed a significantly upregulated mRNA level for zonula occludens-1 (ZO-1) (Fig 2A) and ZO-3 (Fig. 2C); although ZO-2 gene was upregulated to the similar extend, the difference was not statistically significant (Fig. 2B). WSBCW-High also significantly upregulated ZO-1 gene expression (Fig 2A) and non-significantly upregulated expression of ZO-2 and ZO-3 genes.
Fig. 2.
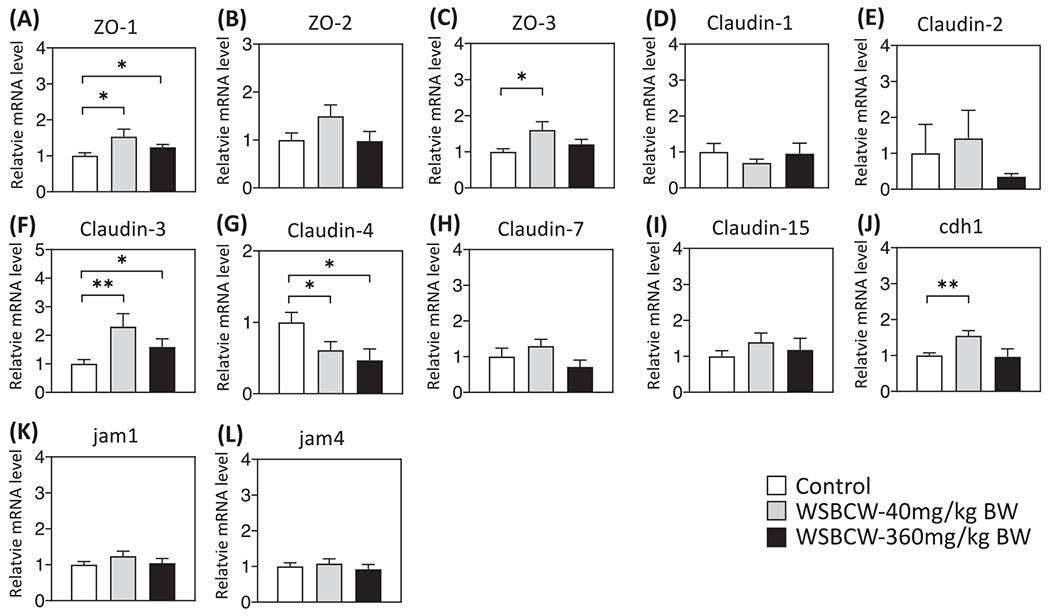
Effects of WSBCW on mRNA levels tight junction and adherens junction genes ZO-1 (A), ZO-2 (B), ZO-3 (C), Claudin-1 (D), Claudin-2 (E), Claudin-3 (F), Claudin-4 (G), Claudin-7 (H), Claudin-15 (I), chd1 (J), jam1 (k) and jam4 (L) in intestine. Within each panel, values with “*” are significantly different from that of the control; **p < 0.01, *p < 0.05.
The mRNA levels of Claudin family members that have high expression levels in the intestine, including Claudin-1, -2, -3, -4, -7, and -15, were compared among groups (Fig. 2D–I). Compared with the control, WSBCW groups showed significantly increased expression of Claudin-3 (Fig. 2F), but significantly decreased expression of Claudin-4 (Fig. 2G). We also investigated the mRNA level of E-cadherin (chd1), a dominant component of adherens junction. The treatment of WSBCW-Low significantly upregulated the mRNA level of cdh1 (Fig. 2J). Besides, we found no significant differences in the mRNA levels of junctional adhesion molecular 1 (JAM1) and JAM4 among experimental groups (Fig. 2K, L). These results suggest that WSBCW modulate intestinal permeability, at least in part, via regulation of tight and adherens junctions.
3.3. WSBCW increased small intestinal villi length
Fig. 3A showed the representative images of H&E staining of the small intestine under indicated treatments. The length of the small intestinal villi was quantified and shown in Fig. 3B. Both WSBCW treatments significantly increased intestinal villi lengths, compared with the control.
Fig. 3.
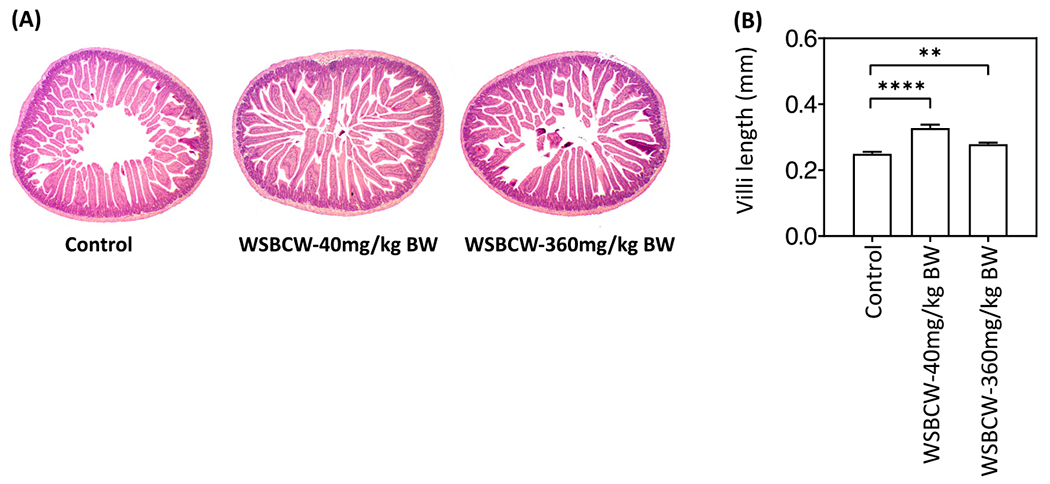
Effects of WSBCW on intestinal villi length. (A) Representative H&E staining images of the small intestine, (B) quantitative results of intestinal villi length of mice under indicated treatments. Values with “*” are significantly different from that of the control; **p < 0.01, ****p < 0.0001.
3.4. WSBCW modulated gut microbiota
We performed a quantitative analysis of eight common gut bacterial species in the cecum of the mice to investigate if/how WSBCW could modulate the gut microbiota community. As shown in Fig. 4, compared with the control group, the WSBCW treatments significantly increased the abundance of Bifidobacterium genus (Fig. 4A), Desulfovibrio genus (Fig. 4B), Atopobium cluster (Fig. 4C) (except the low dose group, apparently due to the higher variation of the results) dose-dependently. Lactobacillus genus showed an increasing trend in a dose-dependent manner, although the differences were not statistically significant (Fig. 4D). Bacteroides fragillis group was significantly decreased by WSBCW dose-dependently (Fig. 4E). The WSBCW treatments did not have significant effects on the abundance of Clostridium coccoides group (Fig. 4F), Clostridium leptum group (Fig 4G), or Prevotella genus (Fig. 4H).
Fig. 4.
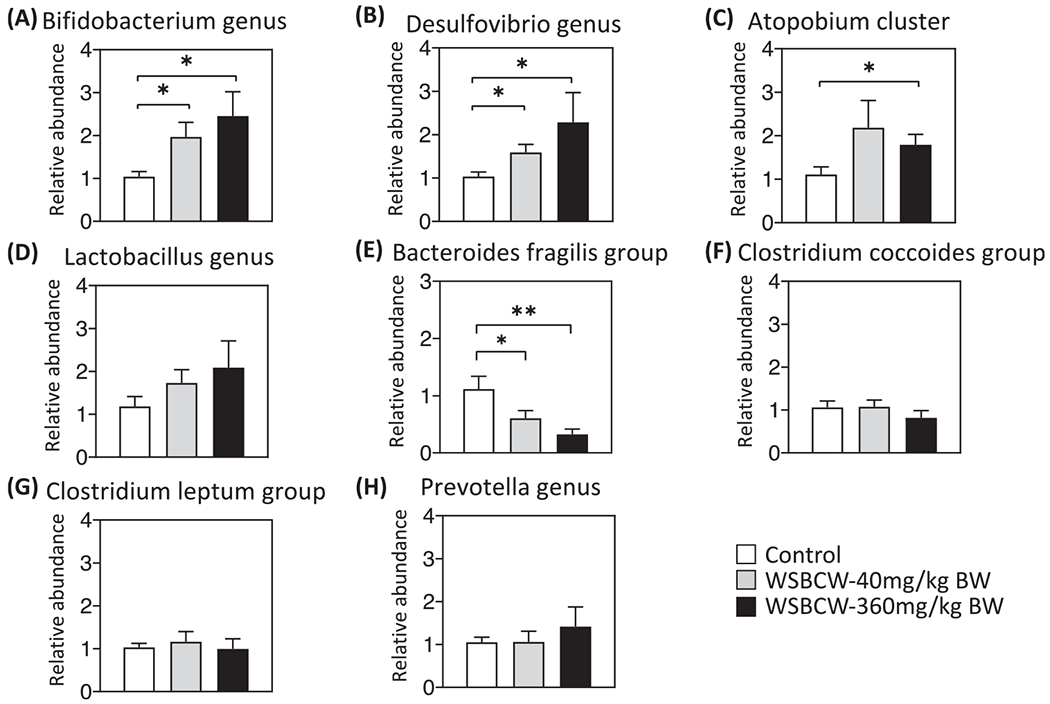
Effects of WSBCW on relative abundance of gut microbiota. (A) Bifidobacterium genus, (B) Desulfovibrio genus, (C) Atopobium cluster, (D) Lactobacillus, (E) genus Bacteroides fragillis group, (F) Clostridium coccoides group, (G) Clostridium leptum group, and (H) Prevotella genus in the cecum of mice in indicated treatment groups. Within each panel, values with “*” are significantly different from that of the control; *p < 0.05, **p < 0.01.
3.5. WSBCW decreased the serum levels of inflammatory cytokines
We detected the serum levels of MCP-1, TNF-α, IL-12 (p40), IL-12 (p70), IL-2, IL-6, and IFN-γ, using the MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel. As shown in Fig. 5, WSBCW significantly decreased the serum levels of TNF-α (Fig. 5A), MCP-1 (Fig. 5B), and IL-12 (p40) (Fig. 5C). IL-6 was decreased under the treatment of WSBCW-High, although not significantly (Fig 5F). Although the WSBCW treatments generally decreased serum levels of IL-12(p70) (Fig. 5D), IL-2 (Fig. 5E), IL-6 (Fig. 5F, except the low dose group) and IFN-γ (Fig. 5G) in a dose-dependent trend, no statistical significances were detected between any experimental groups.
Fig. 5.
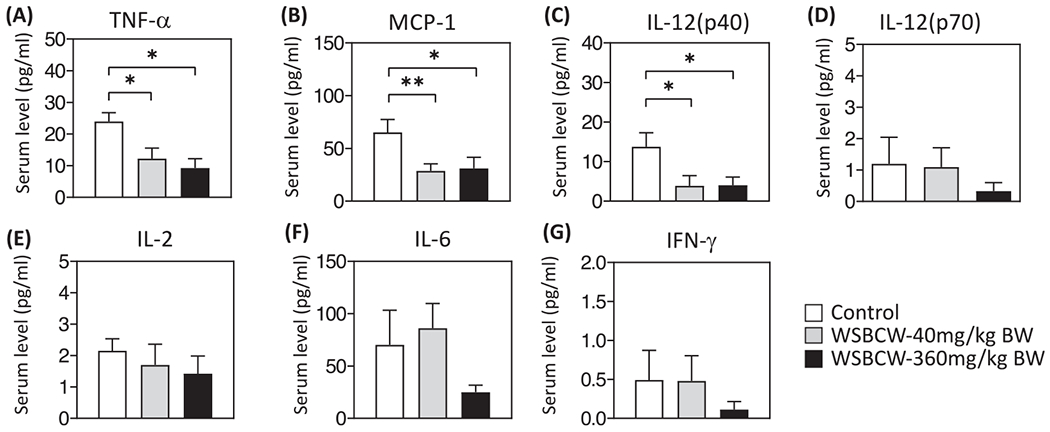
Effects of WSBCW on serum levels of TNF-α(A), MCP-1(B), IL-12 (p40) (C), IL-6 (D), IL-2 (E), IL-12 (p70) (F), and INF-γ(G) in mice of indicated treatment groups. Within each panel, values with “*” are significantly different from that of the control; **p < 0.01, *p < 0.05 compared with control.
3.6. WSBCW effectively inhibited castor oil induced diarrhea
In the study to evaluate the preventive effect of WSBCW on IBS-D, 6 out of 8 mice in the model control group generated watery stool after castor oil administration, with average elution time of 123 ± 28 min, while in the drug control group, 2 out of 8 mice generated watery stool and average of elution time (231 ± 21 min) of watery stool was significantly prolonged. In the WSBCW treatment groups of 40, 120, and 360 mg/kg BW, 3, 4, and 2 out of 8 mice generated watery stool, respectively. Compared with the model control, WSBCW treatments significantly decreased the frequency of mice developing diarrhea (P < 0.05), and also prolonged elution time (Fig. 6A). Specifically, the WSBCW-H treatment significantly prolonged elution time, which was comparable to that of loperamide drug (Fig. 6A).
Fig. 6.
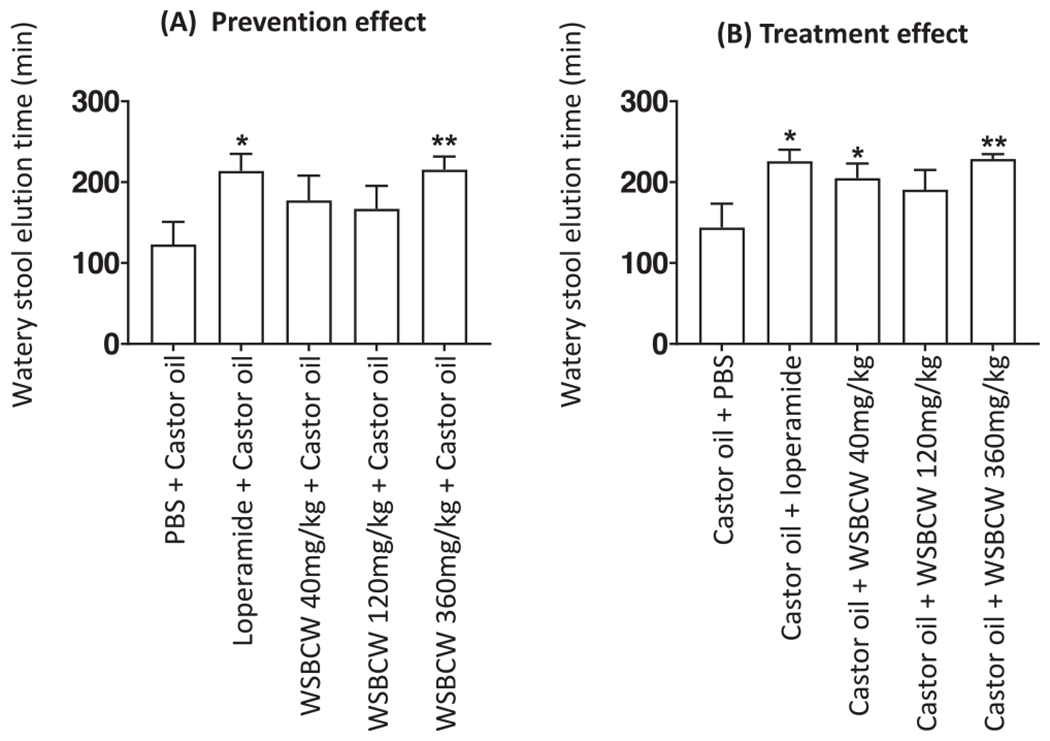
Preventive effect (A) and therapeutic effect (B) of WSBCW on castor oil induced diarrhea. The mice were treated with indicated treatments either before (A) or after (B) administration of castor oil. Time of elution of watery stool was recorded. Within each panel, values with “*” are significantly different from that of the control; **p < 0.01, *p < 0.05.
In the study to evaluate the treatment effect of WSBCW on IBS-D, 8 of 8 mice in the model control group generated water stool after castor oil administration, with an average elution time of 144 ± 27 min, while in the drug control group, 1 of 8 mice generated watery stool and the elution time (221 ± 16 min) of watery stool was significantly prolonged. In the WSBCW treatment groups of 40, 120, and 360 mg/kg BW, 3, 3, and 3 out of 8 mice generated watery stool, respectively. Compared with the model control, WSBCW treatments significantly decreased the frequency of mice developing diarrhea (P < 0.05), and significantly prolonged elution time (Fig. 6B, except for the middle-dose group).
4. Discussion
In the present study, we evaluated the in vivo effects of WSBCW on the regulation of gut movement and intestinal barrier function in healthy conditions and on the prevention and treatment effects on diarrhea in the IBS-D mouse model. WSBCW showed beneficial effects on gut health as demonstrated in increasing whole gut transit and intestinal motility, and reducing intestinal permeability, and increased intestinal villi length in healthy conditions. WSBCW also showed significant preventive and therapeutic effects on diarrhea in the IBS-D model. Biomarker determination showed that WSBCW significantly improved intestinal tight and adherens junctions, inhibited circulating levels of inflammatory cytokines, and modulated gut microbiota. Our results did not show apparent dose-dependent effect, suggesting that WSBCW at the low dose may have sufficient activity.
WSBCW improved intestinal barrier function through regulating the expression of tight junctions and adherens junctions, two main types of cell junctions that are responsible for stitching cells together and neighboring cells, respectively. We investigated mRNA levels of tight junctions, including ZO-1, ZO-2, ZO-3, and members of Claudin family (Claudin-1, -2, -3, -4, -7, -15) that are mainly expressing in the intestine [11], as well as cdh1, jam1, and jam4 that are representative for intestinal adherens junctions [12]. WSBCW significantly upregulated the expression of ZO-1, ZO-3, Claudin-3, and cdh1, the results consistent with the effect of WSBCW on reducing gut permeability. Claudin-2 has been previously reported as a mediator of leaky gut barrier during intestinal inflammation, thus negatively regulates intestinal permeability [13]. In our study, Claudin-2 was slightly downregulated by WSBCW compared with the control group. The reason is probably due to that we used healthy mice for investigation and Claudin-2 level was not activated in the normal, non-inflammatory conditions. Furthermore, we found a significantly downregulated mRNA level of Claudin-4 after the treatment of WSBCW. Although Claudin-4 has been recognized as a tight junction protein, the role of Claudin-4 in regulating intestinal permeability was not well studied. A recent report has indicated that Claudin-4 was dispensable for the intestinal barrier function [14]. If Claudin-4 downregulation contributes to WSBCW effect on improving intestinal barrier function should be further studied in the future research.
On the other hand, previous research has indicated that Claudin-4 may play an essential role in tumor processing and it may serve as a target for anti-cancer therapies [15,16]. Overexpression of Claudin-4 promoted cancer expansion and metastasis of various types of cancer, such as gastric cancer [15], breast cancer [17], and ovarian cancer [18]. Ovarian tumor cell expression of Claudin-4 reduced apoptotic response of ovarian tumor to paclitaxel [19]. Thus, whether or not WSBCW may have anti-cancer activities via downregulating Claudin-4 expression/-function should also be an important topic of future research.
Cdh1, encoding E-cadherin, which plays a central role in intercellular adhesion in the intestinal epithelium, and impaired E-cadherin results in IBD [20,21]. WSBCW upregulated the mRNA level of cdh1, verifying its action mechanism of improving intestinal barrier function and suggesting its potential benefits in IBD treatment.
Diversity and abundance of gut microbiota are critical parameters for the evaluation of gastrointestinal health. Probiotics, such as Bifidobacterium genus and Lactobacillus genus, have been proved to be beneficial for gut health [22]. WSBCW significantly increased the abundance of Bifidobacterium genus in a dose-dependent manner, suggesting that one of the mechanisms by which WSBCW improves gut health is via increasing the abundance of Bifidobacterium genus.
As reported previously, Desulfovibrio genus has a significantly decreased abundance at inflamed site in patients with IBD compared with the corresponding site in non-IBD controls [23]. We found that WSBCW significantly increased Desulfovibrio genus abundance, again suggesting that WSBCW may be beneficial for the treatment of IBD.
Bacteroides fragillis group is one of the most common species in the human intestine. It is capable of synthesizing polysaccharide A (PSA), an agent that modulates the immune system of the host to produce more IL-10 and thus decreases inflammatory level when the PSA gene is on [24]. However, some research groups discovered a subtype of Bacteroides fragillis group that produces a potentially damaging toxin and eventually induces IBD [25]. A higher frequency of Bacteroides fragillis group was reported in IBD patients, especially in IBD-UC, than that in non-IBD groups [25]. Our result showed that WSBCW decreased the abundance of Bacteroides fragillis group significantly. Further investigation is needed to determine if WSBCW can inhibit the enterotoxigenic Bacteroides fragilis and subsequently increase the percentage of Bacteroides fragilis with the PSA gene on, thus benefits for IBD patients.
TNF-α plays a key role in the pathogenesis of IBD, and stimulates production of MCP-1 through pathways, such as NF-κB and p38 MAP kinase activation, in human colonic myofibroblasts [26]. A higher level of proinflammatory chemokine MCP-1 was found in the mucosa of IBD patients compared with the control [27]. Elevated MCP-1 induces a massive migration of monocytes and disturbs the differentiation of intestinal macrophages [28]. IL-12 plays an important role in the development of IBD through a TNF-α independent pathway [29]. Therapy targeting IL-12/23 p40 is used as an alternative for IBD patients that do not respond anti-TNF agents [30]. Our studies showed that WSBCW decreased serum levels of MCP-1, TNF-α, and IL-12 (p40), suggesting that WSBCW may have potent anti-inflammatory activities probably through inhibiting TNF-α and subsequently inhibiting MCP-1. Also, WSBCW significantly decreased IL-12 (p40) level, suggesting its potential role in the therapy of IBD.
5. Conclusions
In conclusion, our experimental results demonstrated that WSBCW significantly improved gastrointestinal health in normal situations as evidenced by increasing whole gut transit and intestinal motility, and reducing intestinal permeability. WSBCW also showed significant preventive and therapeutic effects on diarrhea in the IBS-D model. The effectiveness of WSBCW on gastrointestinal health may be via improved intestinal tight and adherens junctions, inhibited circulating levels of inflammatory cytokines, increased intestinal villi length, and modulated gut microbiota. Our study provided preclinical evidence for verifying the effectiveness of WSBCW in gastrointestinal health and elucidating possible mechanisms of action. The results warrant further preclinical and clinical investigations to evaluate the therapeutic efficacy of WSBCW on gastrointestinal disorders, such as IBS and IBD.
Acknowledgement
This research was funded by Beth Israel Deaconess Medical Center Research Project (#A10164) sponsored by Jinghua Pharmaceuticals Group, China.
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Abbreviations:
- CD
Crohn’s disease
- FL
fluorescence
- FGID
functional gastrointestinal disorders
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- IBS
irritable bowel syndrome
- JAM
junctional adhesion molecule
- MCP-1
monocyte chemotactic protein-1
- PSA
polysaccharide A
- TNF
tumor necrosis factor
- UC
ulcerative colitis
- ZO
zonula occludens
References
- [1].Bischoff SC, ‘Gut health’: a new objective in medicine? BMC Med. 9 (2011) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Noddin L, Callahan M, Lacy BE, Irritable bowel syndrome and functional dyspepsia: different diseases or a single disorder with different manifestations? MedGenMed 7 (3) (2005) 17. [PMC free article] [PubMed] [Google Scholar]
- [3].Chogle A, Velasco-Benitez CA, Koppen IJ, Moreno JE, Ramirez Hernandez CR, Saps M, A population-based study on the epidemiology of functional gastrointestinal disorders in young children, J. Pediatr 179 (2016) 139–143 e1. [DOI] [PubMed] [Google Scholar]
- [4].Boronat AC, Ferreira-Maia AP, Matijasevich A, Wang YP, Epidemiology of functional gastrointestinal disorders in children and adolescents: a systematic review, World J. Gastroenterol 23 (21) (2017) 3915–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Avramidou M, Angst F, Angst J, Aeschlimann A, Rossler W, Schnyder U, Epidemiology of gastrointestinal symptoms in young and middle-aged Swiss adults: prevalences and comorbidities in a longitudinal population cohort over 28 years, BMC Gastroenterol. 18 (1) (2018) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dale HF, Rasmussen SH, Asiller ÖÖ, Lied GA, Probiotics in irritable bowel syndrome: an up-to-date systematic review, Nutrients 11 (9) (2019) 2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Masuy I, Pannemans J, Tack J, Irritable bowel syndrome: diagnosis and management, Minerva Gastroenterol. Dietol 66 (2) (2020) 136–150. [DOI] [PubMed] [Google Scholar]
- [8].Vegh Z, Kurti Z, Lakatos PL, Epidemiology of inflammatory bowel diseases from west to east, J. Dig. Dis 18 (2) (2017) 92–98. [DOI] [PubMed] [Google Scholar]
- [9].Hu SY, Ma R, Guang JX, Feng ZW, Xu HF, Li XM, Bai XH, Ma QP, Xiang XX, Zhu FL, Li WW, Liu PD, Cai JX, Zhang H, Treatment of functional dyspepsia in children with WangshiBaochi Pills: a randomized double-blind multicenter clinical trial, Chin. J. New Drugs 28 (2019) 179–183. [Google Scholar]
- [10].He LX, Abdolmaleky HM, Yin S, Wang Y, Zhou JR, Dietary fermented soy extract and oligo-lactic acid alleviate chronic kidney disease in mice via inhibition of inflammation and modulation of gut microbiota, Nutrients 12 (8) (2020) 2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gunzel D, Yu AS, Claudins and the modulation of tight junction permeability, Physiol. Rev 93 (2) (2013) 525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee SH, Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases, Intest. Res 13 (1) (2015) 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luettig J, Rosenthal R, Barmeyer C, Schulzke JD, Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation, Tissue Barriers 3 (1–2) (2015), e977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tokuda S, Hirai T, Furuse M, Claudin-4 knockout by TALEN-mediated gene targeting in MDCK cells: Claudin-4 is dispensable for the permeability properties of tight junctions in wild-type MDCK cells, PLoS One 12 (8) (2017), e0182521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nishiguchi Y, Fujiwara-Tani R, Sasaki T, Luo Y, Ohmori H, Kishi S, Mori S, Goto K, Yasui W, Sho M, Kuniyasu H, Targeting claudin-4 enhances CDDP-chemosensitivity in gastric cancer, Oncotarget 10 (22) (2019) 2189–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fujiwara-Tani R, Sasaki T, Luo Y, Goto K, Kawahara I, Nishiguchi Y, Kishi S, Mori S, Ohmori H, Kondoh M, Kuniyasu H, Anti-claudin-4 extracellular domain antibody enhances the antitumoral effects of chemotherapeutic and antibody drugs in colorectal cancer, Oncotarget 9 (100) (2018) 37367–37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Duarte GM, Almeida NR, Tocchet F, Espinola J, Barreto CTR, Pinto GA, Soares FA, Marshall P, Russano de Paiva Silva G, Claudin-4 expression is associated with disease-free survival in breast carcinoma-in-situ: mean follow-up of 8.2 years, Clin. Breast Cancer 18 (5) (2018) e1111–e1116. [DOI] [PubMed] [Google Scholar]
- [18].Martin de la Fuente L, Malander S, Hartman L, Jonsson JM, Ebbesson A, Nilbert M, Masback A, Hedenfalk I, Claudin-4 expression is associated with survival in ovarian cancer but not with chemotherapy response, Int. J. Gynecol. Pathol 37 (2) (2018) 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Breed C, Hicks DA, Webb PG, Galimanis CE, Bitler BG, Behbakht K, Baumgartner HK, Ovarian tumor cell expression of claudin-4 reduces apoptotic response to paclitaxel, Mol. Cancer Res 17 (3) (2019) 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mehta S, Nijhuis A, Kumagai T, Lindsay J, Silver A, Defects in the adherens junction complex (E-cadherin/beta-catenin) in inflammatory bowel disease, Cell Tissue Res. 360 (3) (2015) 749–760. [DOI] [PubMed] [Google Scholar]
- [21].Hermiston ML, Gordon JI, Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin, Science 270 (5239) (1995) 1203–1207. [DOI] [PubMed] [Google Scholar]
- [22].Satokari R, Modulation of gut microbiota for health by current and next-generation probiotics, Nutrients 11 (8) (2019) 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hirano A, Umeno J, Okamoto Y, Shibata H, Ogura Y, Moriyama T, Torisu T, Fujioka S, Fuyuno Y, Kawarabayasi Y, Matsumoto T, Kitazono T, Esaki M, Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis, J. Gastroenterol. Hepatol 33 (2018) 1590–1597. [DOI] [PubMed] [Google Scholar]
- [24].Blandford LE, Johnston EL, Sanderson JD, Wade WG, Lax AJ, Promoter orientation of the immunomodulatory Bacteroides fragilis capsular polysaccharide A (PSA) is off in individuals with inflammatory bowel disease (IBD), Gut Microbes 10 (5) (2019) 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rashidan M, Azimirad M, Alebouyeh M, Ghobakhlou M, Asadzadeh Aghdaei H, Zali MR, Detection of B. fragilis group and diversity of bft enterotoxin and antibiotic resistance markers cepA, cfiA and nim among intestinal Bacteroides fragilis strains in patients with inflammatory bowel disease, Anaerobe 50 (2018) 93–100. [DOI] [PubMed] [Google Scholar]
- [26].Aomatsu T, Imaeda Η, Takahashi K, Fujimoto T, Kasumi E, Yoden A, Tamai H, Fujiyama Y, Andoh A, Tacrolimus (FK506) suppresses TNF-alpha-induced CCL2 (MCP-1) and CXCL10 (IP-10) expression via the inhibition of p38 MAP kinase activation in human colonic myofibroblasts, Int. J. Mol. Med 30 (5) (2012) 1152–1158. [DOI] [PubMed] [Google Scholar]
- [27].Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C, Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease, J. Pathol 178 (2) (1996) 201–206. [DOI] [PubMed] [Google Scholar]
- [28].Spoettl T, Hausmann M, Herlyn M, Gunckel M, Dirmeier A, Falk W, Herfarth H, Schoelmerich J, Rogler G, Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes, Clin. Exp. Immunol 145 (1) (2006) 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Neurath MF, IL-23 in inflammatory bowel diseases and colon cancer, Cytokine Growth Factor Rev. 45 (2019) 1–8. [DOI] [PubMed] [Google Scholar]
- [30].Shim HH, Chan PW, Chuah SW, Schwender BJ, Kong SC, Ling KL, A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases, JGH Open 2 (5) (2018) 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]


