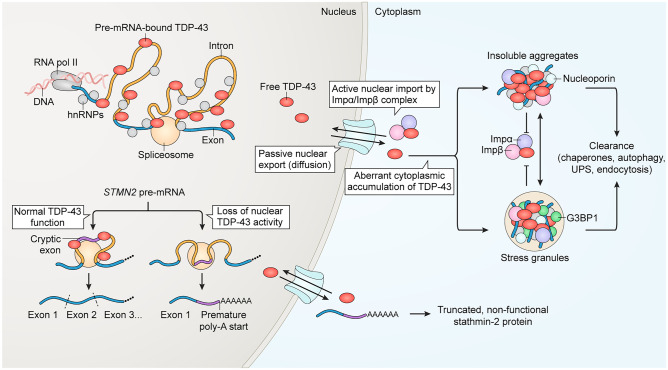
Fig. 1.
Overview of TDP-43 nuclear/cytoplasmic localization and its links to cytoplasmic aggregation and nuclear loss of function in pre-mRNA splicing. In the nucleus, TDP-43 accumulates at GU-rich sequences primarily within the distal introns of thousands of genes, where it acts to modulate splicing. The loss of nuclear TDP-43, triggered by nuclear clearance or disease-causing mutations, unmasks cryptic splice sites that permit the aberrant inclusion of cryptic exons within mRNA. For example, the aberrant inclusion of the STMN2 cryptic exon 2a generates a premature poly-adenylation start site, resulting in the synthesis of non-functional truncated stathmin-2 protein and loss of its crucial function in axonal regeneration. The predominantly nuclear localization of TDP-43 at steady state results from its active nuclear import via importin α and importin β and export via passive diffusion through nuclear pore complexes. In disease, accumulating cytoplasmic TDP-43 forms progressively insoluble aggregates, through a pathway that can proceed by both SG-dependent and -independent pathways. Both TDP-43 aggregates and SGs sequester importins and nucleoporins, which may hamper TDP-43 nuclear re-import. Clearance of cytoplasmic TDP-43 aggregates is mediated by multiple pathways, including via chaperones, autophagy, the ubiquitin–proteasome system (UPS), and endocytosis. For references, see text