Abstract
Hawthorn flavonoids were extracted by enzymolysis associated ultrasonic procedure. Thirteen flavonoids were identified by HPLC/ESI-QTOF/MS, and the major components were procyanidin C1, rutin-rhamnoside, vitexin-rhamnoside, and catechin. Hawthorn flavonoids exhibited strong free radical scavenging activities against DPPH, ABTS, and hydroxyl radicals. Total and intercellular antioxidant experiments revealed that the free hydro-PSC value was 295.32 ± 12.20 μmol of VCE/g DW, and the free cellular antioxidant activity (CAA) values were 168.60 ± 4.87 μmol of QE/g DW in the no PBS wash protocol and 49.53 ± 1.75 μmol of QE/g DW in the PBS wash protocol. In addition, hawthorn flavonoids exhibited higher α-amylase and α-glucosidase inhibitory activities. The wheat starch digestibility was also reduced by hawthorn flavonoids as well. The results indicated that enzymolysis associated ultrasonic extraction was advisable for extracting flavonoids from hawthorn, and hawthorn flavonoids might be recommended as a potential food supplement with hypoglycemic activities.
Keywords: Flavonoids, Antioxidant activity, α-Amylase, α-Glucosidase, Starch digestion
Flavonoids; Antioxidant activity; α-Amylase; α-Glucosidase; Starch digestion.
Highlights
-
•
Ultrasound-enzymolysis combination improved the extraction yield of hawthorn flavonoids.
-
•
Procyanidin C1 and rutin-rhamnoside were major composition of hawthorn flavonoid.
-
•
Hawthorn flavonoids possessed the significant effects on antioxidation and anti-α-amylase, α-glucosidase.
-
•
Hawthorn flavonoids reduced the digestion rate of wheat starch.
1. Introduction
Flavonoids, as the ubiquitous secondary metabolites in the plant, play an important role in health improvement and chronic disease therapy (Yang et al., 2017). Numerous studies have indicated that flavonoids possessed many biological functions, such as antioxidant, anti-inflammation, anti-aging, and immune modulation (Rokayya et al., 2014; Tang et al., 2017; Pérez-Cano et al., 2013). So far, many extraction technologies, e.g. immersion, reflux, ultrasound, microwave, and high hydrostatic pressure extraction have been conducted to extract flavonoids from natural foods (Bimakr et al., 2010; Grigonis et al., 2005; Dahmoune et al., 2014; Briones-Labarca et al., 2015). Among them, the ultrasound-assisted extraction was well confirmed to be the ideal and efficient strategy for the extraction of natural compounds, since it not only be applied to improve the yield, productivity, and safety of products but provides the potential value for extracting bioactive compounds with unique functions from plants (Wen et al., 2018). Besides, the enzymolysis-assisted extraction also has been frequently used in the extraction of neutral components, because it is environmentally compatible and more selective for extracting target compounds from source materials to enhance the yield of phytochemicals (Sowbhagya and Chitra, 2010). However, a single extraction method often needs a long time or consumes a lot of energy but is inefficient (Maier et al., 2008), thereby, the combination extraction methods were well attempted to expect the effective extraction results.
Excess-free radical production has been shown to be associated with cancer, aging, or other diseases (Prochazkova et al., 2011). Therefore, the antioxidant is listed as one of the main research and development directions by health products and cosmetic companies. The antioxidant efficacy of plant flavonoids was often reported, which exert antioxidant effects by inhibiting the production of free radicals, scavenging them directly, or inhibiting lipid peroxidation (Mollica et al., 2018, 2021). Starch is a major energy donor in the daily diet. Rapid digestion of starch can contribute to a rapid increase in postprandial blood glucose levels, which will increase the risk of metabolic diseases such as insulin resistance and diabetes (Ludwig, 2002). The rise in postprandial blood glucose levels can be regulated by controlling the digestion rate of starch. Numerous studies have shown that plant flavonoids have an inhibitory effect on starch digestion, due to the inhibitions of α-amylase and α-glucosidase, which will hinder the contact between starch and amylase to reduce the digestive properties of starch to slow the release of glucose, and in turn decrease the absorption of glucose into the blood (Xu et al., 2020; Li et al., 2021).
Hawthorn (Crataegus pinnatifida) is mostly distributed in China, Europe, and North America. In China, it is cultivated as an edible fruit and approved as a “medicine food homology” fruit by China's National Health Commission (Hou and Jiang, 2013). As a traditional food, it has long been used in preventing and controlling a variety of chronic diseases (Fulton et al., 2016). Hawthorn contains lots of bioactive substances, among which, flavonoids are one of the most important bioactive-component (Pan et al., 2012). However, as far as we know, there is still limited information on the efficient extraction, chemical composition, and biological properties of hawthorn flavonoids.
The objectives of this study were to establish an efficient extraction approach to hawthorn flavonoids and evaluate their biological properties such as antioxidant, anti- α-amylase, and α-glucosidase activities, and inhibition of starch digestibility. The chemical composition of hawthorn flavonoids was also identified.
2. Materials and methods
2.1. Materials
The hawthorn fruit (variety of Mengyin dajinxing, the authentication reference number was SZP096/GPSZ0083) collected from the National Germplasm Resources Garden of Hawthorn located in Shenyang Agricultural University, was cut into pieces after removing the core, dried at 50 °C, then crushed and sieved through 40 mesh for use. Pectinase and acarbose were purchased from Sigma-Aldrich (StLouis, USA). Other chemical reagents used were of analytical grade. Table 1 was a list of abbreviations.
Table 1.
Abbreviations list.
| Abbreviations | Full Name |
|---|---|
| PBD | Plackett-Burman design |
| BBD | Box-Behnken design |
| ANOVA | Analysis of variance |
| HPLC/ESI-QTOF/MS | High Performance Liquid Chromatography/Electro spray ionization-Quadrupole-Time of Flight/mass spectrometry |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS+ | 2,2′-Azinobis- (3-ethylbenzthiazoline-6-sulphonate) |
| PSC | rapid peroxyl radical scavenging capacity |
| CAA | Cellular antioxidant activity |
| DCFH | 2′,7′-Dichlorodihydrofluorescein diacetate |
| ABAP | 2,2′-Azobis- (2-amidinopropane) |
| PNPG | p-Nitrophenyl. α-D-glucopyranoside |
| RDS | Rapidly Digestible Starch |
| Slowly Digestible Starch | |
| Resistant Starch |
2.2. Extraction of hawthorn flavonoids
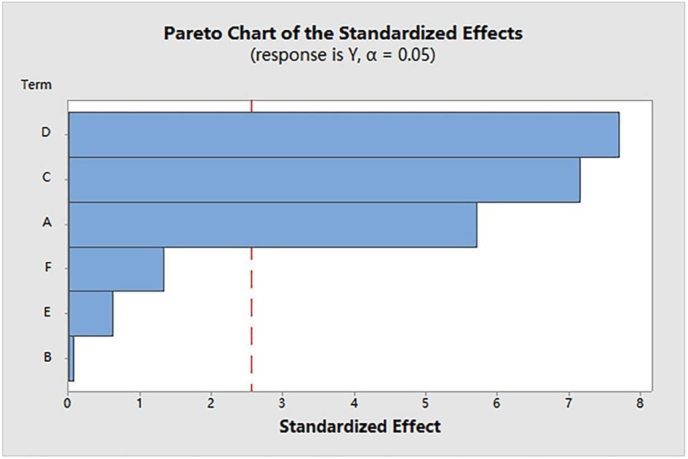
Enzymolysis associated ultrasonic extraction was conducted for extracting flavonoids from hawthorn. In the pre-experiments, six factors which were pectinase catalytic time, pectinase dosage, ultrasonic time, ultrasonic power, liquid to solid ratio, and ethanol concentration were found to significantly affect the yield of hawthorn flavonoids (Figure 1). The most significant factors affecting the flavonoid extraction were selected by Plackett-Burman design (PBD) experiments with the Minitab software (Ver.17.0, USA). Three factors of pectinase dosage, ultrasonic power, and time were confirmed as the most effective variables (Table 2, Figure 2). A Box-Behnken design (BBD) experiment (Design-Expert 8.0.6 Software, Stat-Ease, Inc, USA) was applied for further optimization experiments (Table 3). Each factor was evaluated at three levels: high level (+1), central level (0), and low level (−1). Analysis of variance (ANOVA) was used to analyze the experimental data, the results were fitted by response surface regression. Where predicted response was estimated by Eq. (1) (Chen et al., 2017):
| (1) |
where Y is the predicted response, Xi and Xj are the different independent variables, β0 is the offset term, βi is the ith linear coefficient, βii is the ith quadratic coefficient, and βij is the ijth interaction coefficient (i≠j).
Figure 1.
Effects of main factors on the extraction yields of hawthorn flavonoids. A, pectinase dosage; B, enzymatic time; C, ultrasonic time; D, ultrasonic power; E, ethanol concentration; F, liquid-solid ratio. The different lowercase letters (a–e) indicated significant differences (p < 0.05) among the different samples.
Table 2.
Plackett-Burman desing (PBD) and analysis of variance (ANOVA) for experimental results.
| RunOrder | A | B | C | D | E | F | Y(mg/g) |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | 12.53 |
| 2 | 1 | 1 | 1 | −1 | 1 | 1 | 18.15 |
| 3 | −1 | 1 | 1 | 1 | −1 | 1 | 19.23 |
| 4 | −1 | 1 | 1 | −1 | 1 | −1 | 11.30 |
| 5 | 1 | 1 | −1 | 1 | −1 | −1 | 18.67 |
| 6 | 1 | −1 | 1 | −1 | −1 | −1 | 15.67 |
| 7 | −1 | −1 | 1 | 1 | 1 | −1 | 18.84 |
| 8 | −1 | 1 | −1 | −1 | −1 | 1 | 7.24 |
| 9 | −1 | −1 | −1 | −1 | −1 | −1 | 6.94 |
| 10 | 1 | −1 | −1 | −1 | 1 | 1 | 12.17 |
| 11 | 1 | 1 | −1 | 1 | 1 | −1 | 15.11 |
| 12 | 1 | −1 | 1 | 1 | −1 | 1 | 22.36 |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source | DF | Adj SS | Adj MS | F-Value | P-Value | Inference |
| Model | 6 | 269.936 | 44.989 | 24.22 | 0.002 | ∗∗ |
| Linear | 6 | 269.936 | 44.989 | 24.22 | 0.002 | ∗∗ |
| A | 1 | 60.509 | 60.509 | 32.57 | 0.002 | ∗∗ |
| B | 1 | 0.006 | 0.006 | 0 | 0.956 | |
| C | 1 | 95.139 | 95.139 | 51.21 | 0.001 | ∗∗ |
| D | 1 | 110.304 | 110.304 | 59.38 | 0.001 | ∗∗ |
| E | 1 | 0.704 | 0.704 | 0.38 | 0.565 | |
| F | 1 | 3.273 | 3.273 | 176 | 0.242 | |
| Error | 5 | 9.288 | 1.858 | |||
| Total | 11 | 279.224 | ||||
R2= 0.9667 AdjR2= 0.9268 Pred R2= 0.8084.
A, pectinase dosage (mg/g); B, enzymatic time (min); C, ultrasonic time (min); D, ultrasonic power (W); E, ethanol concentration (%); F, solid-liquid ratio (w/v). Y, the extraction yield of hawthorn flavonoid (mg/g); DF, Degrees of Freedom; Adj SS, Adjusted Sum of Square; Adj MS, Adjusted Mean Square; R2, The coefficient of determination; “∗” and “∗∗”, significant in P < 0.05 and P < 0.01, respectively.
Figure 2.
Pareto chart showed the standardized effect of each variable on the extraction yield of hawthorn flavonoid. A, pectinase dosage; B, enzymatic time; C, ultrasonic time; D, ultrasonic power; E, ethanol concentration; F, liquid-solid ratio. The line in the chart represents a reference line, any factor that extends past this line is of significant effect at P < 0.05.
Table 3.
Box-Behnken desing (BBD) and analysis of variance (ANOVA) for experimental results.
| Run order | X1 | X2 | X3 | Y (mg/g) |
|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 18.28 |
| 2 | 0 | 0 | 0 | 21.11 |
| 3 | 0 | 1 | 1 | 19.84 |
| 4 | −1 | 1 | 0 | 16.85 |
| 5 | 1 | 0 | −1 | 19.15 |
| 6 | −1 | −1 | 0 | 14.01 |
| 7 | −1 | 0 | 1 | 19.20 |
| 8 | 0 | 1 | −1 | 19.18 |
| 9 | 0 | 0 | 0 | 21.35 |
| 10 | −1 | 0 | −1 | 16.12 |
| 11 | 0 | 0 | 0 | 21.51 |
| 12 | 1 | −1 | 0 | 15.01 |
| 13 | 0 | −1 | 1 | 18.42 |
| 14 | 0 | 0 | 0 | 21.01 |
| 15 | 0 | −1 | −1 | 14.23 |
| 16 | 0 | 0 | 0 | 20.56 |
| 17 | 1 | 0 | 1 | 19.51 |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source | SS | df | MS | F-Value | Prob > F | Inference |
| Model | 96.80 | 9 | 10.76 | 84.19 | <0.0001 | ∗∗ |
| X1 | 4.17 | 1 | 4.17 | 32.64 | 0.0007 | ∗∗ |
| X2 | 19.52 | 1 | 19.52 | 152.77 | < 0.0001 | ∗∗ |
| X3 | 8.57 | 1 | 8.57 | 67.08 | < 0.0001 | ∗∗ |
| X1X2 | 0.047 | 1 | 0.047 | 0.37 | 0.5645 | |
| X1X3 | 1.85 | 1 | 1.85 | 14.50 | 0.0066 | ∗∗ |
| X2X3 | 3.11 | 1 | 3.11 | 24.31 | 0.0017 | ∗∗ |
| X12 | 21.25 | 1 | 21.25 | 166.36 | < 0.0001 | ∗∗ |
| X22 | 33.54 | 1 | 33.54 | 262.49 | < 0.0001 | ∗∗ |
| X32 | 0.56 | 1 | 0.56 | 4.42 | 0.0736 | |
| Residual | 0.89 | 7 | 0.13 | |||
| Lack of Fit | 0.36 | 3 | 0.12 | 0.91 | 0.5127 | |
| Pure Error | 0.53 | 4 | 0.13 | |||
| Cor Total | 97.70 | 16 | ||||
R2= 0.9908 Adj R2= 0.9791 Pred R2= 0.9322.
X1: pectinase dosage (mg/g), X2: ultrasonic power (W), X3: ultrasonic time (min), Y: the extraction yield of hawthorn flavonoid (mg/g); DF: Degrees of Freedom, SS: Sum of Square, MS: Mean Square. R2: The coefficient of determination; “∗” and “∗∗”, significant in P < 0.05 and P < 0.01, respectively.
The yield was calculated as the weight (g) of flavonoids obtained from hawthorn fruit (g, DW) and expressed as a percentage.
2.3. Determination of total flavonoids content
Flavonoid content was determined by Jia et al. (1999). Briefly, 1 mL diluted sample solution was added to 0.4 mL of 10% NaNO2 and mixed for 6 min. 0.4 mL of 10% AlCl3 and 1 mol/L NaOH were then successively added and reacted for 6 min, respectively. The absorbance of the reaction solution at 510 nm was measured, and the flavonoid content was calculated by the standard curve using rutin as a standard. The extraction yield was expressed as the ratio of flavonoid to hawthorn powder mass (mg/g).
2.4. HPLC/ESI-QTOF/MS analysis
Hawthorn flavonoids were identified by HPLC/ESI-QTOF/MS (1260 HPLC-6530QTOF, Agilent, USA). A Thermo AQUASIL C18 column (4.6 × 250 mm, 5 μm particle size, USA) was used for the separation at 30 °C. Phase A was 0.1% formic acid in the water, and phase B was 0.1% formic acid in acetonitrile. The gradient programmed was 0–55 min of 95%–0% phase A, and 55–60 min of 100% phase B. The flow rate was 0.4 mL/min, and the injection volume was 2μL. The MS parameters were ESI (−) 100–1500 m/z in full scan mode, gas flow 12 L/min, 300 °C, nebulizer pressure 30 psi, Vcap 3500 V, and fragment voltage 150 V. HPLC/ESI-QTOF/MS data were gathered and processed with Agilent MassHunter software (versions B.06.00).
2.5. Antioxidant activity assay
2.5.1. Measurement of DPPH, ABTS+ and hydroxyl free radical scavenging activities
DPPH and hydroxyl free radical scavenging activities were measured on the basis of our previous methods (Li et al., 2014).
The ABTS+ radical scavenging activity was determined based on the method of Scioli et al. (2022) and slightly modified. A 0.8 mL sample was mixed with 0.2 mL ABTS radical working solution in the dark and absorbance was measured at 734 nm after 6 min reactions. The scavenging activity was calculated as Eq. (2) (Scioli et al., 2022):
| (2) |
where A0 is the Abs of the control, Ai is the Abs of the sample solution.
2.5.2. Determination of the total antioxidant activity by rapid peroxyl radical scavenging capacity (PSC) assay
The PSC assay was conducted as described by Adom and Liu (2005). Briefly, 100 μL of DCFH dye (13.26 μM) was mixed into 100 μL sample solutions. After adding 50 μL ABAP (40 mM), the reaction time was 40 min at 37 °C. Fluorescent was recorded at 485 nm excitation and 538 nm emission. Gallic acid and ascorbic acid were used as standards. The results were calculated as l mol ascorbic acid equivalents per gram of hawthorn fruit on a dry weight basis (l mol VCE/g DW).
2.5.3. Cellular antioxidant activity (CAA) assay
The CAA assay was conducted following the protocol described by Wolfe and Liu (2008). Briefly, after 24 h incubation of HepG2 cells at 37 °C, the growth medium was taken away, and the cells were washed with 100 μL PBS, then treated with 100 μL treatment medium containing hawthorn flavonoids (with 50 μM DCFH-DA) for 1 h. Cells were then treated with or without PBS wash, respectively. 100 μL of oxidant treatment medium (600 μM ABAP) was added to the cells and cultured at 37 °C. At 538 nm emission and 485 nm excitation, the fluorescence was monitored every 5 min for 1 h. CAA was calculated as Eq. (3) (Wolfe and Liu, 2008):
| (3) |
where ʃ SA is the sample fluorescence versus time curve, and ʃ CA is the control in the fluorescence curve.
2.6. Enzyme inhibitory activity assays
2.6.1. α-Glucosidase inhibition assay
α-glucosidase inhibitory activity was evaluated by the method of Zaidi et al. (2019). 50 μL sample solution was mixed with 50 μL enzyme solution at 37 °C for 10 min, then 50 μL PNPG (7.5 mM) was added and incubated at 37 °C for 30 min. Finally, added 100 μL of sodium carbonate (0.1 mol/L), and the absorbance was determined at 405 nm. Acarbose was used as a positive control, and PBS instead of the α-glucosidase solution was used as a blank. The inhibition percentage was calculated as Eq. (4) (Zaidi et al., 2019):
| (4) |
2.6.2. α-Amylase inhibition assay
α-Amylase inhibitory was performed according to the method described by Kwon et al. (2008). Briefly, 0.5 mL sample and 0.5 mL 0.02 mol/L sodium phosphate buffer (pH 6.9 with 6 mM NaCl) containing 0.5 mg/mL α-amylase were incubated at 37 °C for 10 min. Thereafter, 1mL of 1% starch solution was added and incubated at 37 °C for 15 min 1 mL DNS reagent was added to stop the reaction. The reaction mixture was further incubated in a boiling water bath for 5 min, diluted with 10 ml distilled water after cooling. The absorbance was measured at 540 nm, and the inhibition percentage was calculated as Eq. (5) (Kwon et al., 2008):
| (5) |
2.7. In vitro digestibility of wheat starch
Wheat starch (100 mg, dry weight) was dispersed in 4 mL of 0.1 M in sodium acetate buffer (pH 5.2 with 6.67 mmol/L CaCl2) containing 0, 0.024, 0.048, 0.12, 0.24 mg hawthorn flavonoids extracts and 1 ml of α-amylase and glycosylase mixture (40:1). The mixture was incubated at 37 °C and 260 r/min. 0.05 mL reaction solution was withdrawn at 20 min intervals and added to 0.95 mL ethanol to stop the hydrolysis reaction. The glucose content of the supernatant was measured after centrifugation (10,000 rpm, 5 min). The first-order rate equation was fitted by plotting the percentage of hydrolyzed starch Eq. (6) (Li et al., 2021):
| (6) |
where Ct is the amount of starch digested at time t (min), C∞ is the digested starch amount at the end of the reaction, and k(min−1) is the first-order rate coefficient. The calculation formula of content of each starch is as Eqs. (7), (8), and (9) (Patindol et al., 2010):
| (7) |
| (8) |
| (9) |
where, RDS, rapidly digestible starch; SDS, slowly digesting starch; RS, resistant starch; FG (mg), free glucose content before enzymatic hydrolysis. G20 and G120 (mg) were the converted amount of glucose produced by enzymatic hydrolysis of starch within 20 and 120 min, respectively.
2.8. Statistical analysis
Data were expressed as mean ± standard deviation (SD) of three parallel measurements. SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. In all statistical analyses, p < 0.05 were regarded as statistically significant and p < 0.01 as very significant.
3. Results
3.1. Extraction of hawthorn flavonoids
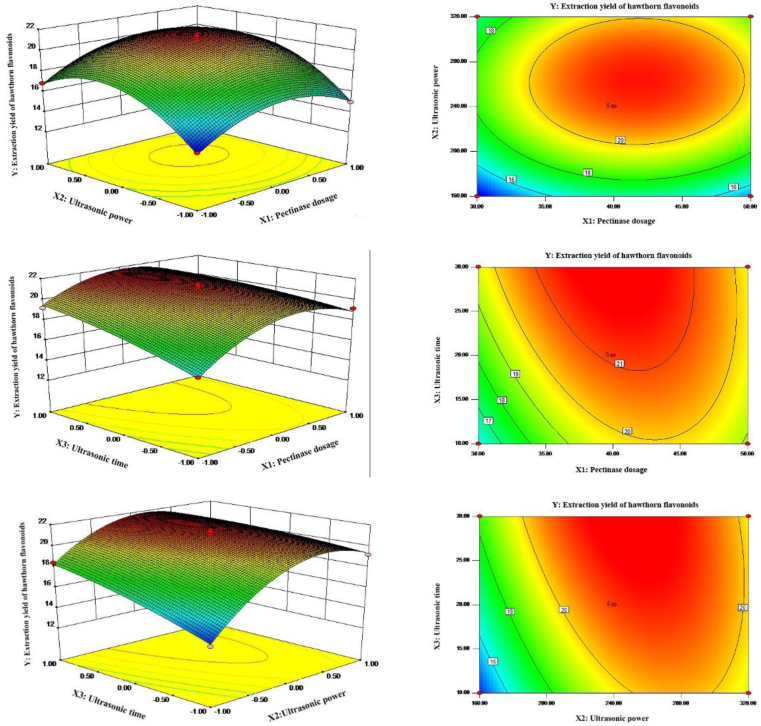
Enzymolysis associated ultrasonic extraction of hawthorn flavonoids was optimized by response surface methodology. As shown in Table 3, the linear term (X1, X2, X3), the quadratic term (X12, X22), and the interaction term (X1X3, X2X3) were significant for the yield of flavonoids. Through multiple regression analysis of experimental data, the equation of extraction rate of hawthorn flavonoid (Y) was obtained as follow: Y = 21.11 + 0.72X1 +1.56X2 + 1.04X3 + 0.11 × X1 × X2 − 0.68 × X2 × X3 − 0.88 × X2 × X3 − 2.25X12 − 2.82X22 − 0.37X32.
In addition, the predicted R2 was 0.9322, which was in good agreement with the adjusted R2 of 0.9791, indicating that the predicted value was consistent with the observed value. According to the regression equation, the response surface curve of X1, X2, and X3 factors to the yields of hawthorn flavonoids was shown in Figure 3. The response surface of X2 and X3 were steep, indicating that the interaction has a significant impact on the yield of flavonoids The optimal extraction conditions for hawthorn flavonoids were calculated as pectinase dosage (X1) 40.11 mg/g, ultrasonic power (X2) 249.6 W, and ultrasonic time (X3) 30 min. In order to facilitate operation, the optimal process parameters were adjusted to a pectinase dosage of 40 mg/g, ultrasonic power of 250 W, and ultrasonic time of 30 min. Under these conditions, the yield was confirmed as 21.2 ± 0.2 mg/g, which was close to the theoretical prediction value of 21.82 mg/g. The results showed that the model was practical and reliable for the optimization of the extraction process of hawthorn flavonoids.
Figure 3.
Response surface plots and contour plots showing the effect of pectinase dosage (X1) ultrasonic power (X2) and ultrasonic time (X3) on yield.
3.2. HPLC/ESI-QTOF/MS analysis
Identification of hawthorn flavonoids was carried out on the basis of HPLC, and molecular and fragment ions in the MS spectrum. 15 main peaks were separated in the HPLC chromatogram (Figure 4) and their structures were identified and listed in Table 4. Among them, 13 components were flavonoids, and the major flavonoids were procyanidin C1, rutin-rhamnoside, vitexin-rhamnoside, and catechin, with the relative amounts of 24.68%, 23.90%, 13.70%, and 8.13%, respectively.
Figure 4.
Chromatographic profile of HPLC/ESI-QTOF/MS for flavonoids from hawthorn.
Table 4.
Retention time and HPLC/ESI-QTOF/MS parameters of the component.
| Peak | Name | Formula | Mw | Fragment (m/z) | Yield (mg/g, DW) |
|---|---|---|---|---|---|
| 1 | Catechin | C15H14O6 | 290 | 289 (M-H)-, 245, 179, 137 | 1.723 |
| 2 | Procyanidin B2 | C30H26O12 | 578 | 577(M-H)-, 289, 865 | 0.644 |
| 3 | Epicatechin | C15H14O6 | 290 | 325(M + Cl)-, 289(M-H)-, 245, 179 | 0.284 |
| 4 | Procyanidin C1 | C45H38O18 | 866 | 865(M-H)-, 577, 289 | 5.232 |
| 5 | Rutin-rhamnoside | C33H40O20 | 756 | 755(M-H)-, 591, 300, 271 | 5.067 |
| 6 | vitexin-glucoside | C27H30O15 | 594 | 611(M-H)+H2O−, 593, 431, 269 | 0.269 |
| 7 | vitexin-rhamnoside | C27H30O14 | 578 | 577(M-H)-, 413, 353 | 2.904 |
| 8 | Rutin | C27H30O16 | 610 | 609(M-H)-, 301 | 1.198 |
| 9 | Vitexin | C21H20O10 | 432 | 431(M-H)-, 415, 313 | 0.420 |
| 10 | Hyperoside | C21H20O12 | 464 | 463(M-H)-, 301 | 0.943 |
| 11 | Isoquercitrin | C21H20O12 | 464 | 463(M-H)-, 301 | 0.435 |
| 12 | Quercetin | C15H10O7 | 302 | 301(M-H)-, 273, 179, 151 | 0.284 |
| 13 | Kaempferol | C15H10O6 | 286 | 258(M-H)-, 257, 185, 151 | 0.269 |
3.3. Antioxidant activities of hawthorn flavonoids
3.3.1. DPPH, ABTS+ and hydroxyl radical scavenging activities of hawthorn flavonoids
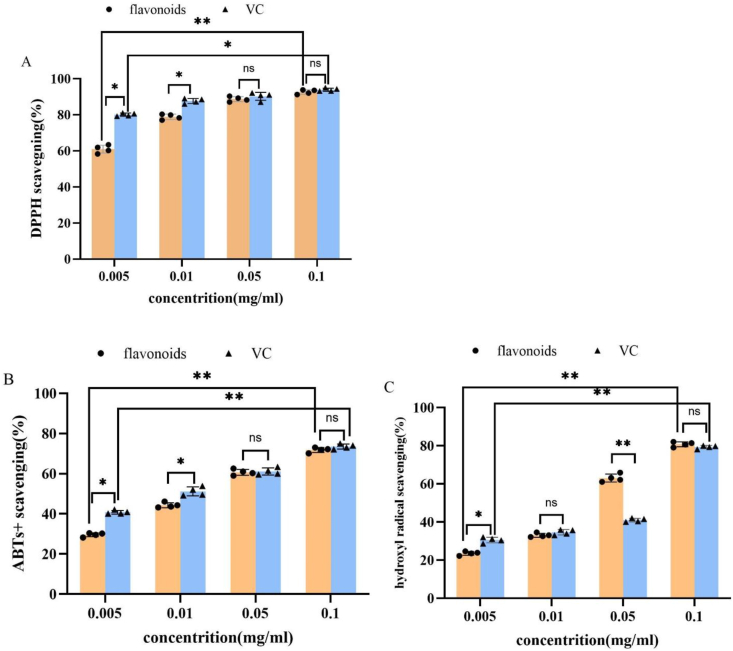
As shown in Figure 5, hawthorn flavonoids exhibited obvious scavenging activities against DPPH, ABTS+, and hydroxyl radicals in a concentration-dependent manner. The scavenging activity against hydroxyl radical in high concentration (0.1 mg/mL) was much higher than that of lower concentrations (0.005–0.05 mg/mL). Moreover, the scavenging activity against DPPH radicals was much higher than that of corresponding concentrations against ABTS+ and hydroxyl radicals, indicating that the hawthorn flavonoid was more sensitive to DPPH radicals. The antioxidant activities against three radicals at higher concentrations (0.05–0.1 mg/mL) were also well comparable to that of Vitamin C.
Figure 5.
Antioxidant activities of hawthorn flavonoids. Data represent means ± SD of three independent experiments. A, DPPH scavenging; B, ABTs+ scavenging; C, hydroxyl radical scavenging. The difference significance was examined with t-test and expressed as: ∗: p < 0.05; ∗∗: p < 0.01; ns: p > 0.05.
3.3.2. Total antioxidant and cellular antioxidant activities of hawthorn flavonoids
PSC assay for hawthorn flavonoids was displayed in Table 5. The data showed that hawthorn flavonoids were responsible for the great peroxyl radical scavenging activity. CAA test (Table 5 and Figure 6) showed that hawthorn flavonoids also possessed strong intercellular antioxidant activity. There was a negative correlation between EC50 and CAA values, indicating a lower EC50 value was consistent with a higher CAA value. The CAA value in the no PBS wash protocol was significantly higher than that in PBS wash, and the cellular uptake values of hawthorn flavonoid was 29.4% expressed as the percentage of CAA values with or without PBS wash.
Table 5.
The EC50, PSC and CAA values.
| Values | EC50 (mg/ml, DW) | |
|---|---|---|
| PSC (μmol of VCE/g, DW) | 325.32 ± 12.20 | 2.835 ± 0.12 |
| CAA (μmol of QE/g, DW, no PBS wash) | 168.60 ± 4.87 | 2.496 ± 0.59 |
| CAA (μmol of QE/g, DW, PBS wash) | 49.53 ± 1.75 | 8.497 ± 1.25 |
Figure 6.
Peroxyl radical-induced oxidation of DCFH to DCF in HepG2, and the inhibition of oxidation by hawthorn flavonoids over time, using the protocol involving no PBS wash and the protocol with a PBS wash. The curves shown in each graph are from a single experiment (mean ± SD, n = 3).
3.4. Inhibition of α-glucosidase, α-amylase, and wheat starch digestion of hawthorn flavonoids
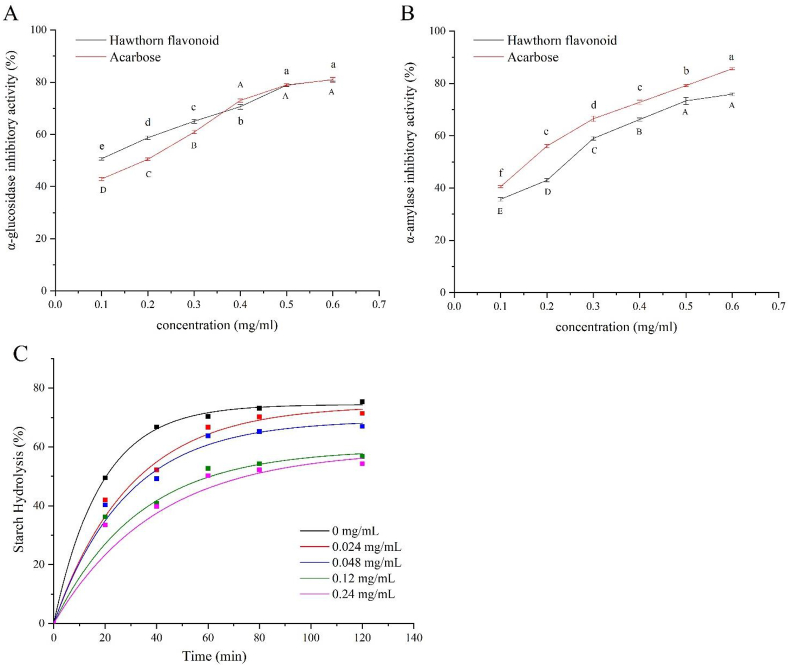
As represented in Figure 7A, hawthorn flavonoids displayed a concentration-dependent α-glucosidase inhibitory activity. The IC50 of hawthorn flavonoids was about 0.112 mg/mL, which was more effective than that of positive control acarbose (IC50 = 0.159 mg/mL). Moreover, hawthorn flavonoids reached their inhibitory activity against α-glucosidase up to 80.97 ± 0.56% at 0.6 mg/mL.
Figure 7.
α-Glucosidase inhibition activity (A); α-amylase inhibition activity (B), and in vitro digestion plots of wheat starch (C) with different concentrations of hawthorn flavonoids.
Similar to the inhibitory tendencies mentioned above, the inhibitory activity of hawthorn flavonoids against α-amylase represented the concentration-dependent manner and increased its activity up to 75.9 ± 0.49% at the concentration of 0.6 mg/mL (Figure 7B). The inhibitory IC50 was 0.207 mg/mL. The inhibitory behavior of hawthorn flavonoids was also similar to a positive control of acarbose.
Based on the results mentioned above, the effect of hawthorn flavonoids on starch digestion was further investigated. As shown in Figure 7C, hawthorn flavonoids significantly reduced the digestion rate of wheat starch. Correspondently, the content of RDS was significantly decreased, and RS was significantly increased with the increase of hawthorn flavonoids (Table 6). Moreover, the C∞ and k decreased from 74.42% and 0.05473 min−1 to 53.77% and 0.04497 min−1, respectively, with the increase of hawthorn flavonoids, indicating that hawthorn flavonoids were able to effectively inhibit the starch hydrolysis (Table 7).
Table 6.
Effects of hawthorn flavonoids on the digestion of wheat starch.
| Hawthorn flavonoids (mg/ml) | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| 0 | 44.55 ± 0.12c | 23.22 ± 0.08b | 32.23 ± 0.04e |
| 0.024 | 37.80 ± 0.23b | 26.47 ± 0.12c | 35.73 ± 0.11d |
| 0.048 | 36.27 ± 0.21b | 24.01 ± 0.09b | 39.72 ± 0.13c |
| 0.12 | 32.58 ± 0.48ab | 18.52 ± 0.23a | 48.89 ± 0.26b |
| 0.24 | 30.15 ± 0.31a | 18.72 ± 0.14a | 51.13 ± 0.17a |
All values are expressed as means ± standard deviation. Values with different letters in the same row indicated a significant difference (p < 0.05).
Table 7.
Kinetic Constant (k) and C∞ of wheat starch in the presence of different concentrations of hawthorn flavonoids extract.
| Hawthorn flavonoids Concentration (mg/mL) | C∞ (%) | k (min−1) | R2 |
|---|---|---|---|
| 0 | 74.42 ± 0.61c | 0.05473 ± 0.0019c | R2 = 0.99902 |
| 0.024 | 72.02 ± 0.21bc | 0.04959 ± 0.0002b | R2 = 0.9999 |
| 0.048 | 67.34 ± 0.56b | 0.04693 ± 0.0014b | R2 = 0.9991 |
| 0.12 | 55.81 ± 0.76ab | 0.04548 ± 0.0027b | R2 = 0.9975 |
| 0.24 | 53.77 ± 1.1a | 0.04497 ± 0.0033a | R2 = 0.9956 |
Values are means ± SD. Mean values with different letters in the column are significantly different (P < 0.05).
4. Discussion
Flavonoids are conventionally extracted from plants with ethanol solution (Biesaga, 2011). However, the dense tissue structure and the cell wall will block its dissolving (Valachovic et al., 2001), thus affecting the extraction efficiency. Ultrasound-assisted extraction has been widely used in the extraction of biologically active substances, because of its capillary and enhanced effects on mass transfer and cell disruption (Shirsath et al., 2012). It is said that ultrasonic extraction is capable to reduce the extraction time and prevent the destruction of the thermolabile flavonoids, thus retaining the desirable properties of flavonoids (Ahmad-Qasem et al., 2013). The use of pectinase to hydrolyze pectin in the cell wall also disrupted the cell wall structure and released intracellular components (Sheldon and Pelt, 2013), which also can enhance the extraction yield of flavonoids from plants (Nadar et al., 2018). In this study, we found that the yield of flavonoids extracted from hawthorn fruit was significantly improved by the combination of enzymolysis-assisted ultrasonic extraction. This synergy effect might be due to the combined action of random destruction of the cell wall by ultrasound and selective destruction of pectinase (Lei et al., 2019).
Generally, the content and composition of hawthorn flavonoids are dependent on the variety and origin (Liu et al., 2010). B-type procyanidins have been reported in hawthorn (Yang and Liu., 2012). Quercetin and kaempferol also have been reported as the flavonol aglycons in hawthorn (Cui et al., 2006). In this study, we have found and identified B2 and C1 type proanthocyanidins, which are mainly polymerized by epicatechin as a flavan-3-ol unit. Meanwhile, monomer epicatechin and catechin as flavonols also existed in hawthorn. In addition, researchers have reported that the distribution of c-glycosyl flavonoids in hawthorn may be an important chemical taxonomic marker for the identification of hawthorn species (Yang and Liu., 2012). Our present study also identified two c-glycosyl flavonoids of vitexin-rhamnoside and vitexin in hawthorn, in agreement with the above suggestion. Beyond that, in the total of 13 hawthorn flavonoids in the present study, procyanidin C1, rutin-rhamnoside, vitexin-rhamnoside, and catechin were quantitatively main components, and it was worth noting that rutin-rhamnoside as the main component in hawthorn had rarely been reported in the past.
On the other hand, oxidative stress caused by free radicals was closely related to a variety of human diseases, such as cardiovascular diseases, autoimmune diseases, and age-related functional decline (Chen et al., 2019). Flavonoids are well known to possess the free radicals scavenging activities against ABTS, DPPH, and hydroxyl radicals as found in this study. The present study further found that hawthorn flavonoids also exhibited strong intercellular antioxidant activities evidenced by the CAA assay, which had a simulation advantage of cellular biochemical processes of the antioxidants (Guo et al., 2017). Such findings were also similar to the flavonoids from other fruits like blueberry, cranberry, apple, and grape (Wolfe and Liu, 2007). These in vitro and intercellular antioxidant capacities revealed the good applicable approaches for hawthorn flavonoids and hawthorn fruit.
Additionally, the inhibition of enzymes is supposed to be the key curative tactic for current health issues. The natural enzyme inhibitors against α-amylase and α-glucosidase are well used to restrain the digestion of starch and to retard the absorption of glucose in the intestine, thereby preventing or therapy obesity and diabetes-related diseases (Cardullo et al., 2020). Lotter et al. (2019) have found that the flavonoids from R. melanophloeos leave represented a strong α-amylase inhibitory activity. Our present study observed that hawthorn flavonoids exhibited dose-dependent inhibitory effects on α-amylase and α-glucosidase. Especially, the inhibitory activities against α-glucosidase at higher conventions (>0.6 μg/ml) were even superior to some extent to positive controls. Furthermore, the addition of hawthorn flavonoids also inhibited the digestion of wheat starch, represented by decreased the content of RDS and increased the content of RS. The reason might be that hawthorn flavonoids could inhibit the amylase, and also the complex structure was formed between hawthorn flavonoids and wheat starch, which further reduced the digestibility of starch (Flanagan et al., 2015). These results indicated that hawthorn flavonoids had an inhibitory effect on the digestive properties of dextrinized wheat starch, which further suggested that flavonoids have a positive effect on reducing the postprandial glucose index, providing an idea for the prevention of diabetes. Such results also extended the application area of hawthorn flavonoids in the areas of health foods and drugs.
5. Conclusion
The results of this work indicated that enzymolysis associated ultrasonic extraction was an effective and feasible strategy for extracting flavonoids from hawthorn with an extraction yield of 20.2 mg/g. Thirteen flavonoid compounds were identified from hawthorn. In which, four compounds including procyanidin C1, rutin-rhamnoside, vitexin-rhamnoside, and catechin were found as major components. The hawthorn flavonoids possessed significant effects on in vitro and cellular antioxidants, and exhibited dose-dependent inhibitory effects on α-amylase and α-glucosidase. It also was able to inhibit starch hydrolysis and reduce starch digestibility to glycosidase. These findings provide the theory and technical foundations for the comprehensive application of hawthorn in the food and drug industries.
Declarations
Author contribution statement
Xin Huang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yuanyuan Bian: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Tianzhi Liu: Contributed reagents, materials, analysis tools or data.
Zhuang Xu, Ziqi Song, Fei Wang: Performed the experiments.
Tuoping Li: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Suhong Li: Conceived and designed the experiments.
Funding statement
This work was supported by Liaoning Province Science and Technology Mission Project (2021030219-JH5/104).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Our warm thanks are addressed to the commercialization of the research findings of Shenyang (20-203-5-59).
Contributor Information
Tuoping Li, Email: litp@syau.edu.cn.
Suhong Li, Email: leesuhong@syau.edu.cn.
References
- Adom K.K., Liu R.H. Rapid peroxyl radical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. J. Agric. Food Chem. 2005;53:6572–6580. doi: 10.1021/jf048318o. [DOI] [PubMed] [Google Scholar]
- Ahmad-Qasem M.H., Cánovas J., Barrajón-Catalán E., Micol V., Cárcel J.A., García-Pérez J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var.Serrana) by using power ultrasound. Innovat. Food Sci. Emerg. Technol. 2013;17:120–129. [Google Scholar]
- Bimakr M., Rahman R.A., Taip F.S., Ganjloo A., Salleh L.M., Selamat J., Hamid A., Zaidul I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2010;89(C1):67–72. [Google Scholar]
- Biesaga M. Influence of extraction methods on stability of flavonoids. J. Chromatogr. A. 2011;1218(18):2505–2512. doi: 10.1016/j.chroma.2011.02.059. [DOI] [PubMed] [Google Scholar]
- Briones-Labarca V., Plaza-Morales M., Giovagnoli-Vicuña C., Jamett F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: effects of extraction conditions and methods. LWT–Food Sci. Technol. 2015;60(1):525–534. [Google Scholar]
- Cardullo N., Muccilli V., Pulvirenti L., Cornu A., Pouységu L., Deffieux D., Quideau S., Tringali C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: a study of α-glucosidase and α-amylase inhibition. Food Chem. 2020;313 doi: 10.1016/j.foodchem.2019.126099. [DOI] [PubMed] [Google Scholar]
- Chen S.H., Huang H.L., Huang G.L. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019;140:1047–1053. doi: 10.1016/j.ijbiomac.2019.08.203. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhang Q., Fei S., Gu H., Yang L. Optimization of ultrasonic circulating extraction of samara oil from Acer saccharum using combination of Plackett-Burman design and Box-Behnken design. Ultrason. Sonochem. 2017;35:161–175. doi: 10.1016/j.ultsonch.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Cui T., Nakamura K., Tian S., Kayahara H., Tian Y. Polyphenolic content and physiological activities of Chinese hawthorn extracts. Biosci. Biotechnol. Biochem. 2006;70(12):2948–2956. doi: 10.1271/bbb.60361. [DOI] [PubMed] [Google Scholar]
- Dahmoune F., Spigno G., Moussi K., Remini H., Cherbal A., Madani K. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014;61:31–40. [Google Scholar]
- Flanagan Bernadine, Gidley Mike, Warren Frederick. Rapid quantification of starch molecular order through multivariate modelling of (13)C CP/MAS NMR spectra. Chem. comm. 2015;51(80) doi: 10.1039/c5cc06144j. [DOI] [PubMed] [Google Scholar]
- Fulton S.L., McKinley M.C., Young I.S., Cardwell C.R., Woodside J.V. The effect of increasing fruit and vegetable consumption on overall diet: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2016;56(5):802–816. doi: 10.1080/10408398.2012.727917. [DOI] [PubMed] [Google Scholar]
- Grigonis D., Venskutonis P.R., Sivik B., Sandahl M., Eskilsson C.S. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloë odorata) J. Supercrit. Fluids. 2005;33(3):223–233. [Google Scholar]
- Guo R.X., Guo X.B., Li T., Fu X., Liu R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017;221:997–1003. doi: 10.1016/j.foodchem.2016.11.063. [DOI] [PubMed] [Google Scholar]
- Hou Y., Jiang J.G. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013;4(12):1727–1741. doi: 10.1039/c3fo60295h. [DOI] [PubMed] [Google Scholar]
- Jia Z.S., Tang M.C., Wu J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- Kwon Y.I., Apostolidis E., Shetty K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008;32(1):15–31. [Google Scholar]
- Lei X., Hu W.B., Zhan W., Yang H.C., Wang N., Liu X., Wang W.J. Enzymolysis-ultrasonic assisted extraction of flavanoid from Cyclocarya paliurus (Batal) Iljinskaja:HPLC profile, antimicrobial and antioxidant activity. Ind. Crop. Prod. 2019;130:615–626. [Google Scholar]
- Li T.P., Li S.H., Dong Y.P., Zhu R.G., Liu Y.H. Antioxidant activity of penta-oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014;145:335–341. doi: 10.1016/j.foodchem.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Li X., Cai J., Yu J., Wang S., Copeland L., Wang S. Inhibition of in vitro enzymatic starch digestion by coffee extract. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129837. [DOI] [PubMed] [Google Scholar]
- Liu P.Z., Yang B.R., Kallio H. Characterization of phenolic compounds in Chinese hawthorn (Crataegus pinnatifida Bge. var. major) fruit by high performance liquid chromatography–electrospray ionization mass spectrometry. Food Chem. 2010;121(4):1188–1197. doi: 10.1016/j.foodchem.2011.01.103. [DOI] [PubMed] [Google Scholar]
- Lotter N., Chivandi E., Lembede B.W., Ndhlala A.R., Nyakudya T.T., Erlwanger K.H. Anti-oxidant activity, alpha-amylase inhibition and toxicity of leaf extracts of cultivated Rapanea melanophloeos (L.) Mez (cape beech) South Afr. J. Bot. 2019;126:261–264. [Google Scholar]
- Ludwig D.S. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Maier T., Göppert A., Kammerer D.R., Schieber A., Carle R. Optimization of a process for enzyme assisted pigment extraction from grape (Vitis vinifera L.) pomace. Europ. Food Res. Technol. 2008;227(1):267–275. [Google Scholar]
- Mollica A., Scioli G., Della Valle A., Cichelli A., Novellino E., Bauer M., Kamysz W., Llorent-Martinez E.J., Luisa Fernandez-de Cordova M., Castillo-Lopez R., Ak G., Zengin G., Pieretti S., Stefanucci A. Phenolic analysis and in vitro biological activity of red wine, pomace and grape seeds oil Derived from Vitis vinifera L. Cv. Montepulciano d'Abruzzo. Antioxidants. 2021;10(11) doi: 10.3390/antiox10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A., Stefanucci A., Zengin G., Locatelli M., Macedonio G., Orlando G., Ferrante C., Menghini L., Recinella L., Leone S., Chiavaroli A., Leporini L., Di Nisio C., Brunetti L., Tayrab E., Ali I., Musa T.H., Musa H.H., Ahmed A.A. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018;107:129–138. doi: 10.1016/j.biopha.2018.07.169. [DOI] [PubMed] [Google Scholar]
- Nadar S.S., Rao P., Rathod V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: a review. Food Res. Int. 2018;108:309–330. doi: 10.1016/j.foodres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Pan G.Y., Yu G.Y., Zhu C.H., Qiao J.L. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS) Ultrason. Sonochem. 2012;19(3):486–490. doi: 10.1016/j.ultsonch.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Patindol J.A., Guraya H.S., Champagne E.T., McClung A.M. Nutritionally important starch fractions of rice cultivars grown in Southern United States. J. Food Sci. 2010;75:H137–H144. doi: 10.1111/j.1750-3841.2010.01627.x. [DOI] [PubMed] [Google Scholar]
- PerezCano F.J., Franch A., PerezBerezo T., Ramos-Romero S., Castellote C., Castell M. In: Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases. Watson R.R., Preedy V.R., editors. Academic Press; 2013. Chapter 12-the effects of flavonoids on the immune System; pp. 175–188. [Google Scholar]
- Prochazkova D., Bousova I., Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Rokayya S., Li C.J., Zhao Y., Li Y., Sun C.H. Cabbage (Brassica oleracea L. var.capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. APJCP. 2014;14(11):6657–6662. doi: 10.7314/apjcp.2013.14.11.6657. [DOI] [PubMed] [Google Scholar]
- Scioli G., Della Valle A., Zengin G., Locatelli M., Tartaglia A., Cichelli A., Stefanucci A., Mollica A. Artisanal fortified beers: Brewing, enrichment, HPLC-DAD analysis and preliminary screening of antioxidant and enzymatic inhibitory activities. Food Biosci. 2022;48 [Google Scholar]
- Sheldon R.A., Pelt S.V. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 2013;42(15):6223–6235. doi: 10.1039/c3cs60075k. [DOI] [PubMed] [Google Scholar]
- Shirsath S.R., Sonawane S.H., Gogate P.R. Intensification of extraction of natural products using ultrasonic irradiations—a review of current status. Chem. Eng. Process: Process Intensif. 2012;53:10–23. [Google Scholar]
- Sowbhagya H.B., Chitra V.N. Enzyme assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010;50(2):146–161. doi: 10.1080/10408390802248775. [DOI] [PubMed] [Google Scholar]
- Tang W.Z., Wang Y.A., Gao T.Y., Wang X.J., Zhao Y.X. Identification of C-geranylated flavonoids from Paulownia catalpifolia Gong Tong fruits by HPLC-DAD-ESI-MS/MS and their anti-aging effects on 2BS cells induced by H2O2. Chin. J. Nat. Med. 2017;15(5):384–391. doi: 10.1016/S1875-5364(17)30059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valachovic P., Pechova A., Mason T.J. Towards the industrial production of medicinal tincture by ultrasound assisted extraction. Ultrason. Sonochem. 2001;8(2):111–117. doi: 10.1016/s1350-4177(00)00066-3. [DOI] [PubMed] [Google Scholar]
- Wen C.T., Zhang J.X., Zhang H.H., Dzah C.S., Zandile M., Duan Y.Q., Ma H.L., Luo X.P. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrason. Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Wolfe K.L., Liu R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008;56(18):8404–8411. doi: 10.1021/jf8013074. [DOI] [PubMed] [Google Scholar]
- Wolfe K.L., Liu R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007;55(22):8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhou J., Yu J., Wang S., Copeland L., Wang S. Revealing the mechanisms of starch amylolysis affected by tea catechins using surface plasmon resonance. Int. J. Biol. Macromol. 2020;145:527–534. doi: 10.1016/j.ijbiomac.2019.12.161. [DOI] [PubMed] [Google Scholar]
- Yang B., Liu P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012;92(8):1578–1590. doi: 10.1002/jsfa.5671. [DOI] [PubMed] [Google Scholar]
- Yang X., Wang Q., Pang Z.R., Pan M.R., Zhang W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in c57bl/6 mice. Pharmaceut. Biol. 2017;55(1):1207–1214. doi: 10.1080/13880209.2016.1278454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi H., Ouchemoukh S., Amessis-Ouchemoukh N., Debbache N., Pacheco R., Luisa Serralheiro M., Eduarda Araujo M. Biological properties of phenolic compound extracts in selected Algerian honeys—the inhibition of acetylcholinesterase and α-glucosidase activities. Europ. J. Integ. Med. 2019;25:77–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.