Abstract
We investigated the ability of copeptin level to predict adverse outcome in pediatric heart failure (HF) and correlated copeptin level with various clinical and echocardiographic data. This cohort study was carried out on forty children with clinical picture of acute HF as the patient group and forty healthy children of matched age and sex as the control group. Echocardiographic examination and plasma copeptin level were performed for all included children at admission. Patients were followed up for 6 months for mortality or readmission. Plasma copeptin level was significantly higher in the patient group (16.2 ± 5) pmol/L compared to the control group (4.1 ± 2.3) pmol/L, P ˂0.001. Moreover, copeptin level was positively correlated with Ross classification, being the highest in patients with class IV (19.6 ± 3.9) pmol/L compared to those with class III (15.2 ± 4) pmol/L and class II (10.4 ± 1.5) pmol/L. Copeptin levels were significantly higher in patients with bad prognosis (21.2 ± 4.1) pmol/L compared to those with good prognosis (14.5 ± 4.1) pmol/L, P ˂0.001. Copeptin level had a significant positive correlation with age, heart rate, respiratory rate, and ROSS classification. On the contrary, copeptin level had a significant negative correlation with left ventricular fraction shortening and diastolic function. Copeptin at cut-off value of ≥ 19.5 pmol/L yielded a sensitivity of 75% and a specificity of 93% to predict adverse outcome in children with HF. Plasma copeptin level has a good prognostic value to predict adverse outcome in pediatric heart failure. Moreover, copeptin correlate well with the severity of pediatric HF.
Keywords: Copeptin, Heart failure, Mortality, Pediatric, Predictive value
Introduction
Heart failure (HF) is one of the most common causes of morbidity and mortality in pediatrics worldwide [1]. Independent of the etiology, neurohormonal activation plays an important role in the pathophysiology of the development and progression of HF [2]. The renin angiotensin aldosterone system (RAAS) as well as vasopressin (VP) and the sympathetic nervous system are activated in cases of HF leading to salt and water retention, vasoconstriction, and increased heart rate [3].
Low cardiac output as well as increased osmolality in HF lead to stimulation of the release of VP hormone from the posterior pituitary which has both antidiuretic and vasoconstrictor properties and thus regulating water balance and hemodynamics of the body [4]. Early prediction of high risk patients with HF who need more intense strategy of management is crucial to improve the survival rate. Several biomarkers have been studied to predict the severity of HF, adverse outcome, and survival in children with HF [5, 6].
Copeptin is synthesized with VP and released in equimolar amounts. It is considered as a surrogate biomarker for VP due to its stability and longer half-life than VP [7]. Plasma copeptin is found to correlate well to VP levels in plasma [8]. Increased copeptin level has been reported in adults with HF [9, 10]. Furthermore, copeptin was reported to have a good prognostic value to predict mortality and adverse outcome in adults with HF in several studies [11, 12]. However, the prognostic value of copeptin in pediatric HF has not been evaluated yet.
The aim of this study was to evaluate the ability of copeptin level to predict adverse outcome in pediatric HF and to correlate copeptin level with various clinical and echocardiographic data.
Patients and Methods
This cohort study was carried out at Pediatric Cardiology unit, Tanta University hospital during the period from January 2019 to December 2020. Forty children with clinical picture of acute HF were enrolled as the patient group. Forty healthy children of matched age and sex served as the control group. Patients were classified according to modified Ross classification of HF in infants and children to class I, II, III, and IV [13]. The study was approved by the local ethical committee of faculty of medicine, Tanta University. Written informed consents were obtained from parents of all children involved in the study.
- Inclusion Criteria
Children aged less than 18 years with manifestations of acute HF either due to acquired or congenital heart disease (CHD).
- Exclusion Criteria
children with neuromuscular mitochondrial disease, ischemic heart disease, cancer, diabetes mellitus, diabetes insipidus, obesity, central nervous system diseases, hepatic, or renal disease.
HF was diagnosed both clinically by symptoms and signs of HF as tachycardia, tachypnea, enlarged tender liver, dyspnea, orthopnea, edema, sweating, and feeding intolerance, and radiologically by chest X ray and echocardiography.
All participants were subjected to full history taking and thorough clinical examination including weight, heart rate (HR), respiratory rate (RR), and complete local cardiac examination. Routine laboratory evaluation including complete blood count, liver function tests, and kidney function tests was performed.
Conventional Echocardiographic examination was performed using a Vivid seven ultrasound machine (GE Medical System, Horten, Norway, with a 3.5 MHz multifrequency transducer) to evaluate left ventricular (LV) systolic function using two-dimensional and M mode at parasternal long axis view. LV systolic function was evaluated as: LV fractional shortening (FS) = LV end-diastolic dimension (LVEDD) − LV end-systolic dimension (LVESD) / (LVEDD) × 100%. Left ventricular diastolic function was measured using pulsed Doppler through the mitral valve in the form of mitral E/A ratio where E wave is a peak early filling velocity and A wave is a peak late filling velocity.
Plasma levels of copeptin was measured using a sandwich enzyme-linked immunosorbent assay test (ELISA). Two milliliters of venous blood sample was collected from each patient in an EDTA vacutainer tube labelled with the patient name. The blood was mixed gently and centrifuged for separation of plasma, which was stored at − 20 °C till the time of analysis. Plasma Copeptin levels were analyzed with an ELISA kit (Shanghai SunRed Bio-Tech. CO. LTD, China). The intra-and inter-assay coefficients of variation (CV) were ˂ 10 and ˂ 12% respectively.
Echocardiographic examination and plasma copeptin levels were measured at the time of admission. Patients were followed up for 6 months for adverse outcomes such as mortality and re-admission to the hospital. Good prognosis was defined as no mortality, readmission, or complications during the period of follow-up, while poor prognosis was defined as the incident of death, readmission, or complications during the period of follow-up. Complications were defined as the occurrence of HF or chest infection.
Statistical analysis
Statistical analysis was performed using SPSS V.20. For quantitative data, the mean and standard deviation (SD) were calculated. For qualitative data, number and percentages were calculated. Comparison of qualitative data between two groups was performed using Chi-square test (χ2). Comparison of the means between the two groups was performed using Student t-test. Comparison of the mean between more than two groups was performed using one way analysis of variance (ANOVA) test. Correlation between variables was evaluated using Pearson’s correlation coefficient (r). The Receiver Operating Characteristic (ROC) curve was drawn to detect the prognostic value of copeptin to predict adverse outcome among children with HF at different cutoff points. P < 0.05 is considered significant.
Results
The study included 40 children with HF as the patient group with mean age 3.8 ± 2.7 years, they were 15 male and 25 female. Forty healthy children of matched age and sex served as the control group with mean age 4 ± 2.9 years, they were 17 male and 23 female. There was no significant difference between the patient and control group as regards age and sex. Weight, LV FS, and mitral E/A ratio were significantly lower in the patient group compared to the control group. HR and RR were significantly higher in the patient group compared to the control group. Thirteen out of the 40 patient (32.5%) had HF due to cardiomyopathy, while 27 out of 40 patients (67.5%) had HF due to CHD. Patients with CHD (27 patients) were diagnosed as; eight patients with ventricular septal defect, five patients with patent ductus arteriosus, seven patients with complete atrioventricular canal, four patients with transposition of great arteries, two patients with coarctation of aorta, one patient with pulmonary stenosis. Plasma copeptin level was significantly higher in the patient group (16.2 ± 5) compared to the control group (4.1 ± 2.3), P ˂0.001. (Table 1).
Table 1.
Demographic, clinical, and laboratory data in the studied groups
| Parameters | Patient | Control | P value |
|---|---|---|---|
| Age (years) | 3.8 ± 2.7 | 4 ± 2.9 | 0.514 |
| Sex (male:female) | 15:25 | 17:23 | 0.648 |
| Weight (kg) | 8.1 ± 2.5 | 9.3 ± 2.2 | 0.028 |
| HR (beat/min) | 116.7 ± 17.9 | 101.9 ± 9.4 | < 0.001 |
| RR (cycle/min) | 40.9 ± 10.2 | 34.6 ± 6.7 | 0.001 |
| LV FS | 19.6 ± 4.2 | 39.3 ± 4.2 | < 0.001 |
| Mitral E/A ratio | 1.1 ± 0.2 | 1.5 ± 0.1 | 0.001 |
| Diagnosis | |||
| Cardiomyopathy | 13 (32.5%) | ||
| CHD | 27 (67.5%) | ||
| ROSS classification | |||
| Class I | 0 (0%) | 40 (100%) | |
| Class II | 7 (17.5%) | 0 (0%) | |
| Class III | 16 (40%) | 0 (0%) | |
| Class IV | 17 (42.5%) | 0 (0%) | |
| Copeptin levels (pmol/L) | 16.2 ± 5 | 4.1 ± 2.3 | < 0.001 |
HR heart rate, RR respiratory rate, LV FS left ventricular fraction shortening, E/A ratio E wave is a peak early filling velocity and A wave is a peak late filling velocity, CHD congenital heart disease
Copeptin level was significantly higher in patients with Ross class IV (19.6 ± 3.9) compared to those with class III (15.2 ± 4) and class II (10.4 ± 1.5), P ˂ 0.001 (Table 2).
Table 2.
Copeptin levels in different Ross classification in the patient group
| Parameter | Class II (n = 7) | Class III (n = 16) | Class IV (n = 17) | P value |
|---|---|---|---|---|
| Copeptin level (pmol/L) | 10.4 ± 1.5 | 15.2 ± 4 | 19.6 ± 3.9 | < 0.001 |
| Post Hoc test | II vs III | II vs IV | III vs IV | |
| P1 = 0.018 | P2 ≤ 0.001 | P3 = 0.004 |
The mortality rate in our study was 7.5% (three patients); readmission within the period of follow-up occurred in another nine patients (22.5%). Moreover, copeptin levels were significantly higher in patients with bad prognosis (21.2 ± 4.1) compared to those with good prognosis (14.5 ± 4.1), P ˂ 0.001. (Table 3).
Table 3.
Copeptin levels in patients with good and bad prognosis in the patient group
| Patients with HF (n = 40) | Number | Percentage (%) | Copeptin level |
|---|---|---|---|
| Patients with bad prognosis | 12 | 30 | 21.2±4.1 |
| Death | 3 | 7.5 | |
| Readmission | 9 | 22.5 | |
| Patients with good prognosis | 28 | 70 | 14.5 ± 4.1 |
| P value | < 0.001 | ||
HF heart failure
Copeptin level had a significant positive correlation with age, HR, RR, and ROSS classification. On the contrary, copeptin level had a significant negative correlation with LV FS and mitral E/A ratio. Moreover, copeptin level had no significant correlation with sex, weight, or diagnosis. (Table 4).
Table 4.
Correlation between copeptin levels and various clinical and echocardiographic data
| Parameters | Copeptin (pmol/L) | |
|---|---|---|
| R | P | |
| Age | 0.247 | 0.027 |
| Sex | 0.089 | 0.431 |
| HR | 0.460 | < 0.001 |
| RR | 0.404 | < 0.001 |
| Weight | −0.050 | 0.661 |
| LV FS | −0.826 | < 0.001 |
| Mitral E/A ratio | −0.811 | < 0.001 |
| Diagnosis | 0.153 | 0.631 |
| Ross classification | 0.897 | ˂ 0.001 |
HR heart rate, RR respiratory rate, LV FS left ventricular fraction shortening, E/A ratio E wave is a peak early filling velocity and A wave is a peak late filling velocity
ROC curve showed that the cut-off value of ≥ 19.5 pmol/L yielded a sensitivity of 75% and a specificity of 93% to predict adverse outcome in children with acute HF. (Fig. 1).
Fig. 1.
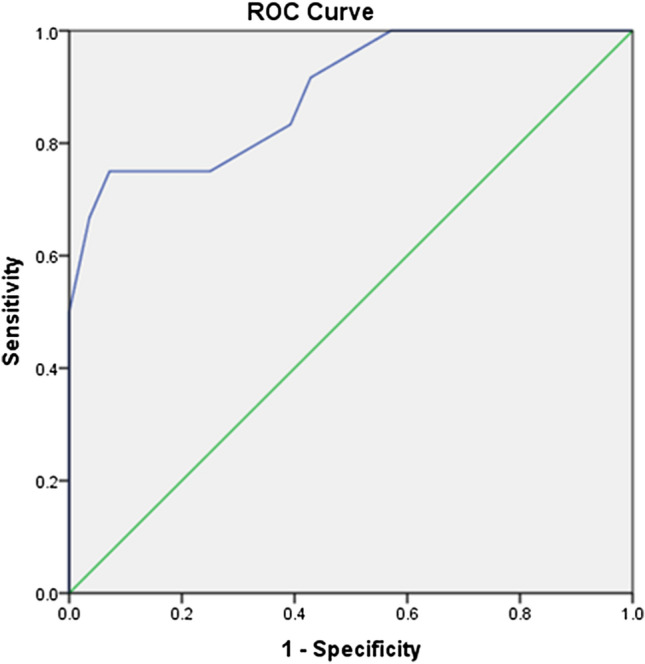
ROC curve to assess the predictive value of copeptin level to predict adverse outcome in children with acute HF
Discussion
The Predictive role of plasma copeptin levels has been studied in several studies in adults. However, its value as a predictive biomarker for HF is still under evaluated in pediatrics.
In our study, we found that the mean copeptin levels were significantly higher in children with HF compared to the control group indicating that copeptin level can be used as a marker for the diagnosis of HF. Similar result was documented in adults with HF [14, 15]. Recently, a preprint study in pediatrics showed that copeptin levels were elevated in children with HF compared to healthy control [16]. Moreover, Karki et al. [17] found that copeptin levels were higher in pediatric patients with HF due to cardiomyopathy compared to the control group. During HF, low cardiac output and atrial pressure stimulate neurohormonal reflexes with a subsequent increase of VP secretion that explain the high level of copeptin in HF [18].
Copeptin level was found to positively correlate with the severity of HF presented by ROSS classification being the highest in patients with class IV and the lowest in patients with class II which reflected the role of copeptin in pathogenesis and identification of the severity of HF. Similar results were reported by other researchers [14, 17]. Increased copeptin level could be used as a marker of more advanced HF and inevitable hospitalization [19].
Moreover, copeptin level was inversely correlated with LV FS and mitral E/A ratio indicating that copeptin level was higher with more advanced HF. Whether it is a cause or effect, this needs more research to investigate this point. In addition to the well-known vasoconstriction and water retention effects of vasopressin, it has been proposed to exert direct effects on the myocardium leading to left ventricular hypertrophy and remodeling with a subsequent negative effects on myocardial contractility [20, 21].
Copeptin level was also found to be positively correlated with age, but whether this was due to the effect of more advanced disease or the effect of the age per se is still unclear. Future studies are required to address this question.
Interestingly, copeptin levels were significantly higher in children with poor prognosis (mortality and readmission) compared to those with good prognosis. Moreover, copeptin at a cut-off value of ≥ 19.5 pmol/L yielded a sensitivity of 75% and a specificity of 93% to predict poor prognosis in children with HF. In line with our results, copeptin was found to be a strong predictor for all case mortality/hospitalization by earlier studies in adults [22–29]. Copeptin serves as a surrogate biomarker for VP levels. Accordingly, elevated plasma levels of copeptin reflect an unfavorable hemodynamic profile and a poor outcome in patients with HF [16, 17, 28].
HF is characterized by a high rate of mortality and morbidity in children thus finding an easy dependable marker for identifying high risk patients who need early intervention and more intense treatment is crucial to improve outcome and to decrease mortality.
Limitation of the study: relatively small number of patients, being a single center study, serial measurement of copeptin level was not performed, and diagnostic value of copeptin level to predict the response of treatment in such patients was not evaluated.
Conclusion
Plasma copeptin level has a good prognostic value to predict adverse outcome in pediatric HF. Moreover, copeptin correlated well with the severity of pediatric HF.
Author Contributions
DE idea of the research, collected the study data, wrote, revised and approved the manuscript. DA performed the laboratory analysis, wrote, revised and approved the manuscript. MN performed the statistical analysis, collected the study data, wrote, revised and approved the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). none.
Data Availability
Available when requested.
Code Availability
Not applicable.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
Local ethics committee of Faculty of Medicine, Tanta University approved the study. The study is in accordance with the ethical standards of institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Consent to Participate
An informed consent was obtained from the parents of all subjects of the study before enrollment.
Consent to Publication
All the authors transfer, assign, or otherwise convey all copyright ownership, including any and all rights exclusively to the journal, in the event that such work is published by the journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Doaa El Amrousy, Email: doaa.moha@med.tanta.edu.eg.
Dina Abdelhai, Email: dinaibraheem85@yahoo.com.
Mohammed Nassar, Email: drnassar2012@gmail.com.
References
- 1.Nandi D, Rossano JW. Epidemiology and cost of heart failure in children. Cardiol Young. 2015;25:1460–1468. doi: 10.1017/S1047951115002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madriago E, Silberbach M. Heart failure in infants and children. Pediatr Rev. 2010;31:4–12. doi: 10.1542/pir.31.1.4. [DOI] [PubMed] [Google Scholar]
- 3.El Amrousy D, Hodeib H, Suliman G, Hablas N, Salama ER, Esam A. Diagnostic and prognostic value of plasma levels of cardiac myosin binding protein-C as a novel biomarker in heart failure. Pediatr Cardiol. 2017;38(2):418–424. doi: 10.1007/s00246-016-1532-2. [DOI] [PubMed] [Google Scholar]
- 4.Schweiger TA, Zdanowicz MM. Vasopressin-receptor antagonists in heart failure. Am J Health Syst Pharm. 2008;65:807–817. doi: 10.2146/ajhp070132. [DOI] [PubMed] [Google Scholar]
- 5.El Amrousy D, Hassan S, Hodeib H. Prognostic value of homocysteine and highly sensitive cardiac troponin t in children with acute heart failure. J Saudi Heart Assoc. 2018;30:198–204. doi: 10.1016/j.jsha.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Amrousy D, El-Mahdy H. Prognostic value of serum apelin level in children with heart failure secondary to congenital heart disease. Pediatr Cardiol. 2018;39:1188–1193. doi: 10.1007/s00246-018-1879-7. [DOI] [PubMed] [Google Scholar]
- 7.Gaheen R, El Amrousy D, Hodeib H, Elnemr S. Plasma copeptin levels in children with pulmonary arterial hypertension associated with congenital heart disease. Eur J Pediatr. 2021;180:2889–2895. doi: 10.1007/s00431-021-04060-9. [DOI] [PubMed] [Google Scholar]
- 8.Morgenthaler NG, Struck J, Jochberger S, Dunser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–49. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Balling L, Gustafsson F. Copeptin in heart failure. Adv Clin Chem. 2016;73:29–64. doi: 10.1016/bs.acc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Wasilewski MA, Myers VD, Recchia FA, Feldman AM, Tilley DG. Arginine vasopressin receptor signaling and functional outcomes in heart failure. Cell Signal. 2016;28:224–233. doi: 10.1016/j.cellsig.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schill F, Timpka S, Nilsson PM, Melander O, Enhorning S. Copeptin as a predictive marker of incident heart failure. ESC Heart Failure. 2021;8:3180–3188. doi: 10.1002/ehf2.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K, for the OPTIMAAL Investigators, C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the optimaal study. Eur Heart J. 2009;30:1187–1194. doi: 10.1093/eurheartj/ehp098. [DOI] [PubMed] [Google Scholar]
- 13.Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. 2012;33(8):1295–1300. doi: 10.1007/s00246-012-0306-8. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Liu X, Wu S, Gai L. The clinical application value of the plasma copeptin level in the assessment of heart failure with reduced left ventricular ejection fraction. Medicine. 2018;97:39. doi: 10.1097/MD.0000000000012610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maisel A, Xue Y, Shah K, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, et al. Increased 90-day mortality in patients with acute heart failure with elevated copeptin: secondary results from the biomarkers in acute heart failure (BACH) study. Circ Heart Fail. 2011;4(5):613–620. doi: 10.1161/CIRCHEARTFAILURE.110.960096. [DOI] [PubMed] [Google Scholar]
- 16.Abdelaziz AA, Khattab AA, Abdelmaksoud MH, Ghazy RM. Serum Copeptin Level as a Predictor Marker of Pediatric Heart Failure Outcomes, 28 September 2021, preprint available at Research Square. 2021 doi: 10.21203/rs.3.rs-917008/v1. [DOI] [Google Scholar]
- 17.Karki KB, Towbin JA, Philip RR, Harrell C, Tadphale S, Shah S, Saini A. Copeptin: a novel biomarker in pediatric heart failure due to cardiomyopathies. Circulation. 2019;140:A11217. [Google Scholar]
- 18.Vinod P, Krishnappa V, Chauvin AM, Khare A, Raina R. Cardiorenal syndrome: role of arginine vasopressin and vaptans in heart failure. Cardiol Res. 2017;8:87–95. doi: 10.14740/cr553w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balling L, Kistorp C, Schou M, Egstrup M, Gustafsson I, Goetze JP, Hildebrandt P, Gustafsson F. Plasma copeptin levels and prediction of outcome in heart failure outpatients: relation to hyponatremia and loop diuretic doses. J Cardiac Fail. 2012;18:351–358. doi: 10.1016/j.cardfail.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–1791. doi: 10.1016/j.jacc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- 21.Kelly D, Squire IB, Khan SQ, Quinn P, Morgenthaler NG, Davies JE, Ng LL. C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling, and clinical heart failure in survivors of myocardial infarction. J Card Fail. 2008;14:739–745. doi: 10.1016/j.cardfail.2008.07.231. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Wu X, Li G, Sun H, Shi J. Prognostic role of copeptin with all-cause mortality after heart failure: a systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:49–58. doi: 10.2147/TCRM.S124689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30(10):1187–1194. doi: 10.1093/eurheartj/ehp098. [DOI] [PubMed] [Google Scholar]
- 24.Peacoc WF, Nowak R, Christenson R, Di Somma S, Neath SX, Hartmann O, Mueller C, Ponikowski P, Mockel M, Hogan C, et al. Short-term mortality risk in emergency department acute heart failure. Acad Emerg Med Off J Soc Acad Emerg Med. 2011;18(9):947–958. doi: 10.1111/j.1553-2712.2011.01150.x. [DOI] [PubMed] [Google Scholar]
- 25.Alehagen U, Dahlström U, Rehfeld JF, Goetze JP. Association of copeptin and N-terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. J Am Med Assoc. 2011;305:2088–2095. doi: 10.1001/jama.2011.666. [DOI] [PubMed] [Google Scholar]
- 26.Masson S, Latini R, Carbonieri E, Moretti L, Rossi MG, Ciricugno S, Milani V, Marchioli R, Struck J, Bergmann A, et al. The predictive value of stable precursor fragments of vasoactive peptides in patients with chronic heart failure: data from the GISSI-heart failure (GISSI-HF) trial. Eur J Heart Fail. 2010;12(4):338–347. doi: 10.1093/eurjhf/hfp206. [DOI] [PubMed] [Google Scholar]
- 27.Neuhold S, Huelsmann M, Strunk G, Stoiser B, Struck J, Morgenthaler NJ, Bergmann A, Moertl D, Berger R, Pacher R. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: prediction of death at different stages of the disease. J Am Coll Cardiol. 2008;52:266–272. doi: 10.1016/j.jacc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 28.Stoiser B, Mortl D, Hulsmann M, Berger R, Struck J, Morgenthaler NJ, Bergmann A, Pacher R. Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Investig. 2006;36(11):771–778. doi: 10.1111/j.1365-2362.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 29.Tentzeris I, Jarai R, Farhan S, Perkmann T, Schwarz MA, Jakl G, Wojta J, Huber K. Complementary role of copeptin and high-sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail. 2011;13(7):726–733. doi: 10.1093/eurjhf/hfr049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available when requested.
Not applicable.


