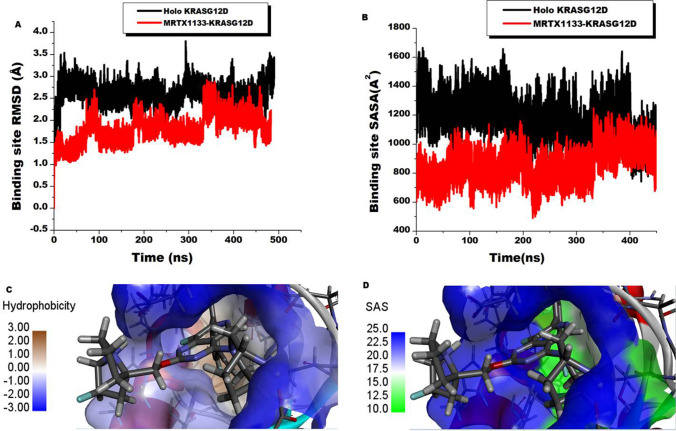
Figure 2.
Comparative stability and solvent accessibility surface area of the binding site of KRASG12D upon the binding of MRTX1133 and the holo protein. (A) Shows the RMSD plots of the C-a atoms of the binding site residues indicating MRTX1133 binding stabilized the residues (red) relative to the holo (black). (B) Shows the SASA plots of the binding site residues indicating a reduction in surface area availability upon MRTX1133 binding (red) relative to the holo protein (black). (C) Shows the hydrophobicity of the binding site. (D) Shows the solvent accessible of area of the binding site.