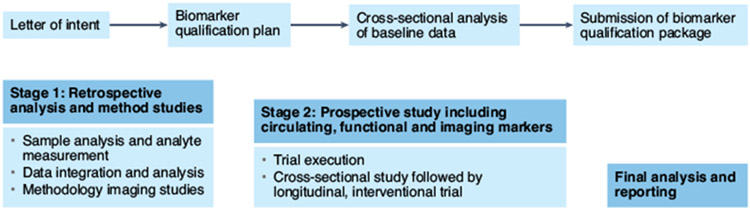
Fig. 1 ∣. The FNIH NIMBLE project plan.
The biomarker qualification process was initiated by approval of the letter of intent submitted by the NIMBLE consortium to the FDA. This will be complemented by cross-sectional studies of circulating biomarkers and methodological studies of MRI and ultrasound-based biomarkers in stage 1. After completion of stage 1, a full biomarker qualification plan will be submitted for review by the FDA. The results of stage 1 will also trigger stage 2, which will include a prospective evaluation of selected biomarkers for final and full qualification combining data from both stage 1 and stage 2.