Figure 3. Interactions between proteasome-associated receptors and ubiquitin processing enzymes.
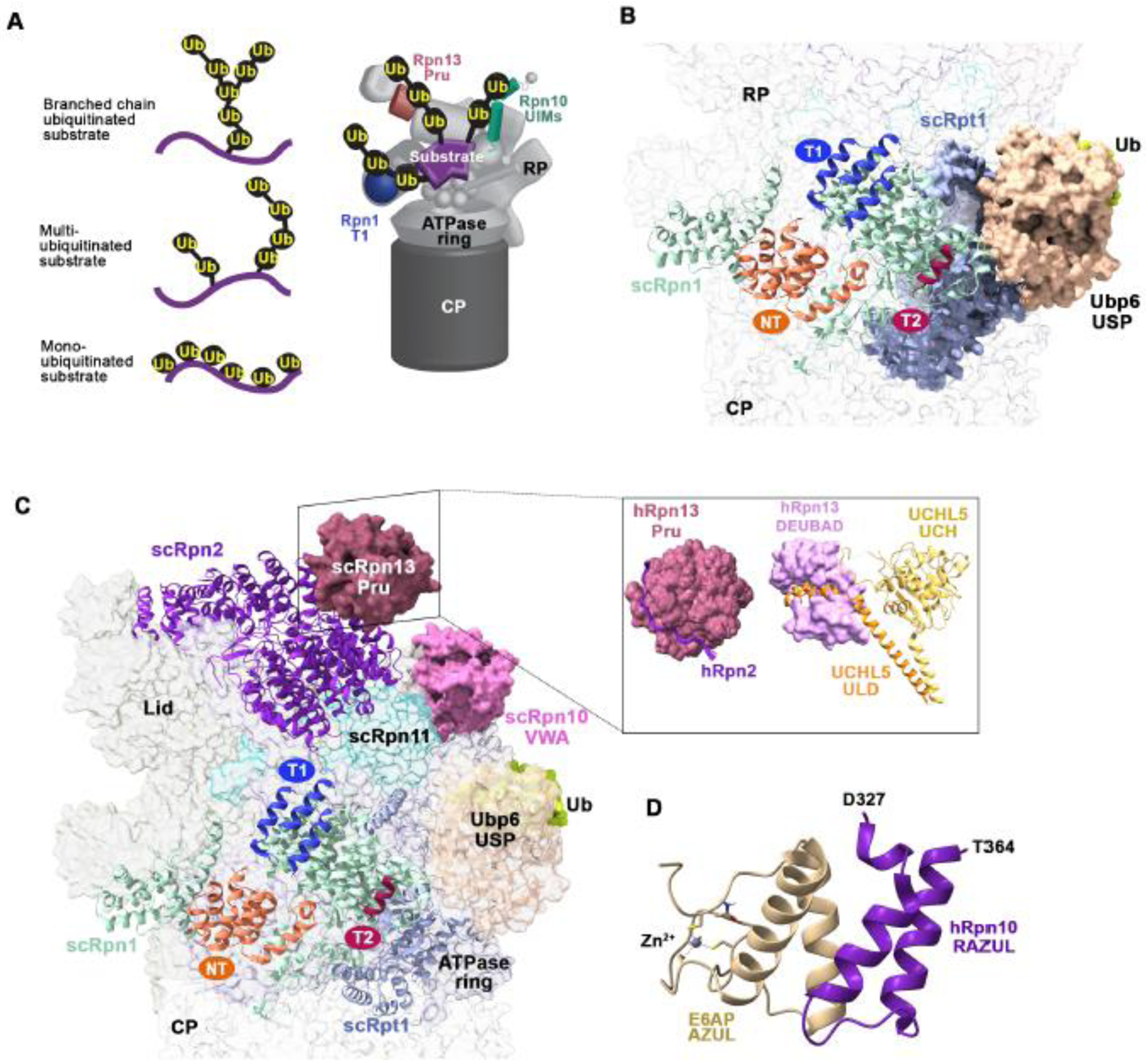
(A) Representation of differentially ubiquitinated substrates including with a single branched ubiquitin chain (top left) or multiubiquitinated substrates with chains (middle left) or monoubiquitin (bottom left) attached. A model of how a multiubiquitinated substrate (purple) may engage the Rpn10 UIMs (green), Rpn13 Pru (pink), and Rpn1 T1 (indigo) is provided (right). (B) View of the Rpn1 and Rpt1 (light blue) regions of the budding yeast 26S proteasome bound to the Ubp6 catalytic domain (USP, light orange) conjugated to ubiquitin aldehyde (green). The Rpn1 (pale green) T1, T2, and NT sites are colored indigo, burgundy, and deep orange, respectively. The Ubp6 N-terminal UBL domain was not visible in this cryo-EM structure (PDB:5A5B). (C) As in (B) but displaying Rpn13 Pru (pink) at Rpn2 (violet) (left; PDB:5A5B). A box insert shows the human domains with interacting partners, including hRpn13 Pru bound to the C-terminus of hRpn2 and the hRpn13 DEUBAD domain (PDB:6CO4) bound to UCHL5 (ULD, orange; catalytic domain (UCH), yellow; PDB:4UEL). (D) Structure of the hRpn10 RAZUL (dark purple) bound to the E6AP AZUL (pale yellow; PDB;6U19). Zinc is displayed as a grey sphere. All structure images were generated with ChimeraX.
