Figure 3.
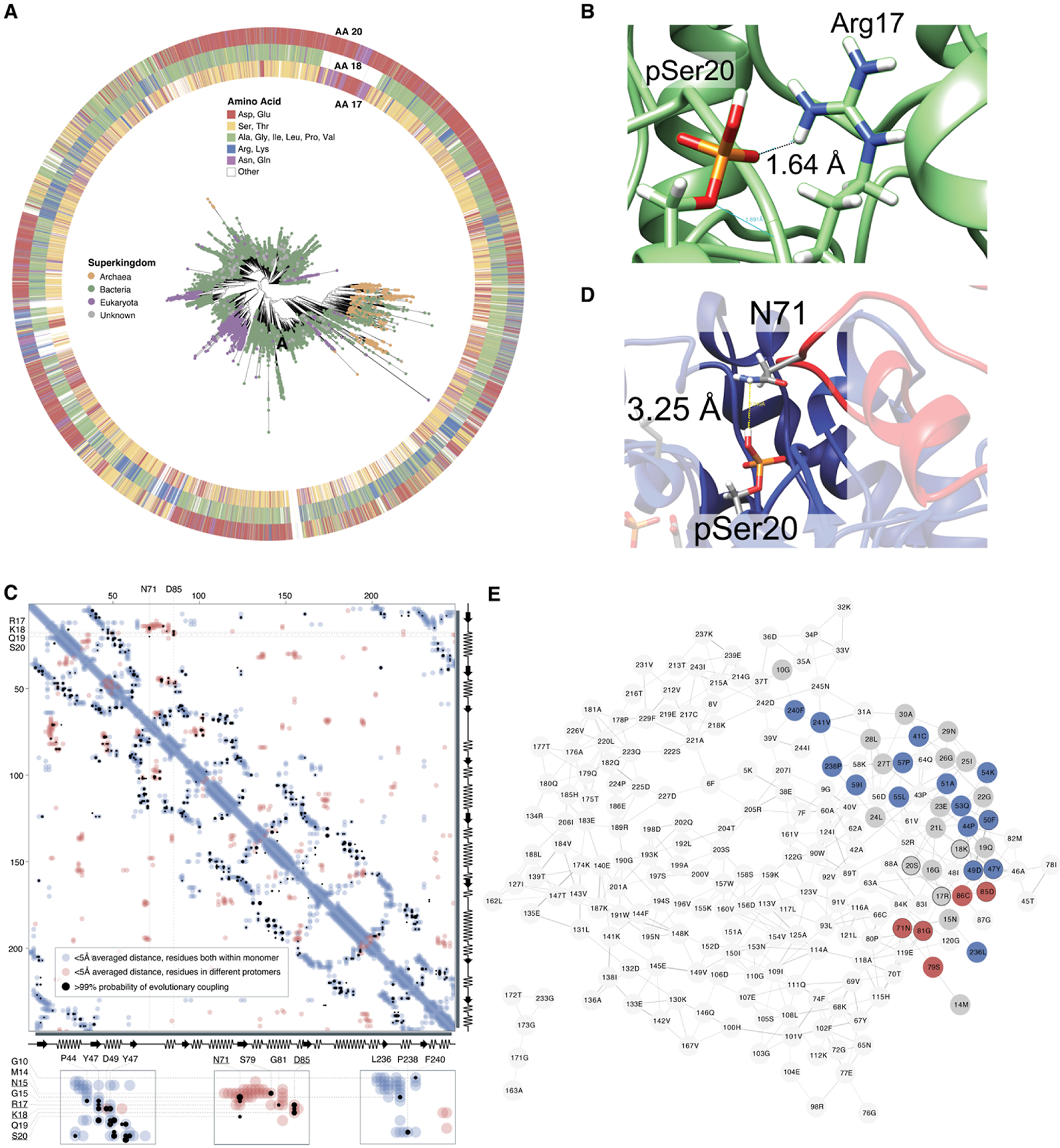
Sequence-based analysis of 54722 TPI sequences across the tree of life. (A) All TPI sequences in the UniRef90 database were used to construct sequence similarity networks (SSNs), and for visualization, representative protein sequences were chosen at 70% identity (Figure S10A–D). These representative sequences were additionally aligned against the TPI Pfam hidden Markov model (PF00121), and this alignment was used to construct an unrooted phylogenetic tree. Nodes are colored according to superkingdom membership, and residue identity at positions 17/18 and 20 is illustrated surrounding the tree. (B) Interaction between Arg17 and pSer20 in our structural model. (C) Evolutionary coupling of residues in TPI. Analyses were carried out using EVCouplings V2, at a bitscore of 0.7, but the same couplings were observed at lower bitscores. Residues within a 5 Å distance within the structure of the monomeric protein are indicated in light blue, while residues on separate protomers within a homomultimeric unit that are within an average 5 Å are highlighted in red. Residues with a >99% likelihood of evolutionary coupling are indicated in black. (D) Notably, despite a predicted distance that may preclude direct hydrogen bonding, Ser20 and Asn71 are proposed as an evolutionarily coupled pair, likely as part of interactions at or near the homodimer interface. Coupling between Arg17, Lys18, and Asp85 is additionally likely to be directly related to the formation of the homodimer interface. (E) Residue co-evolution network for long-distance co-evolutionary partners, organized by connectivity with unweighted edges (edges represent >99% likelihood for evolutionary coupling). Residues with intramolecular proximity to Asn15-Ile25 are shown in gray; more distant residues with evolutionary coupling on the same subunit as Asn15-Ile25 are highlighted in blue; distant residues on the subunit opposite to Asn15-Ile25 are highlighted in red.
