Figure 4.
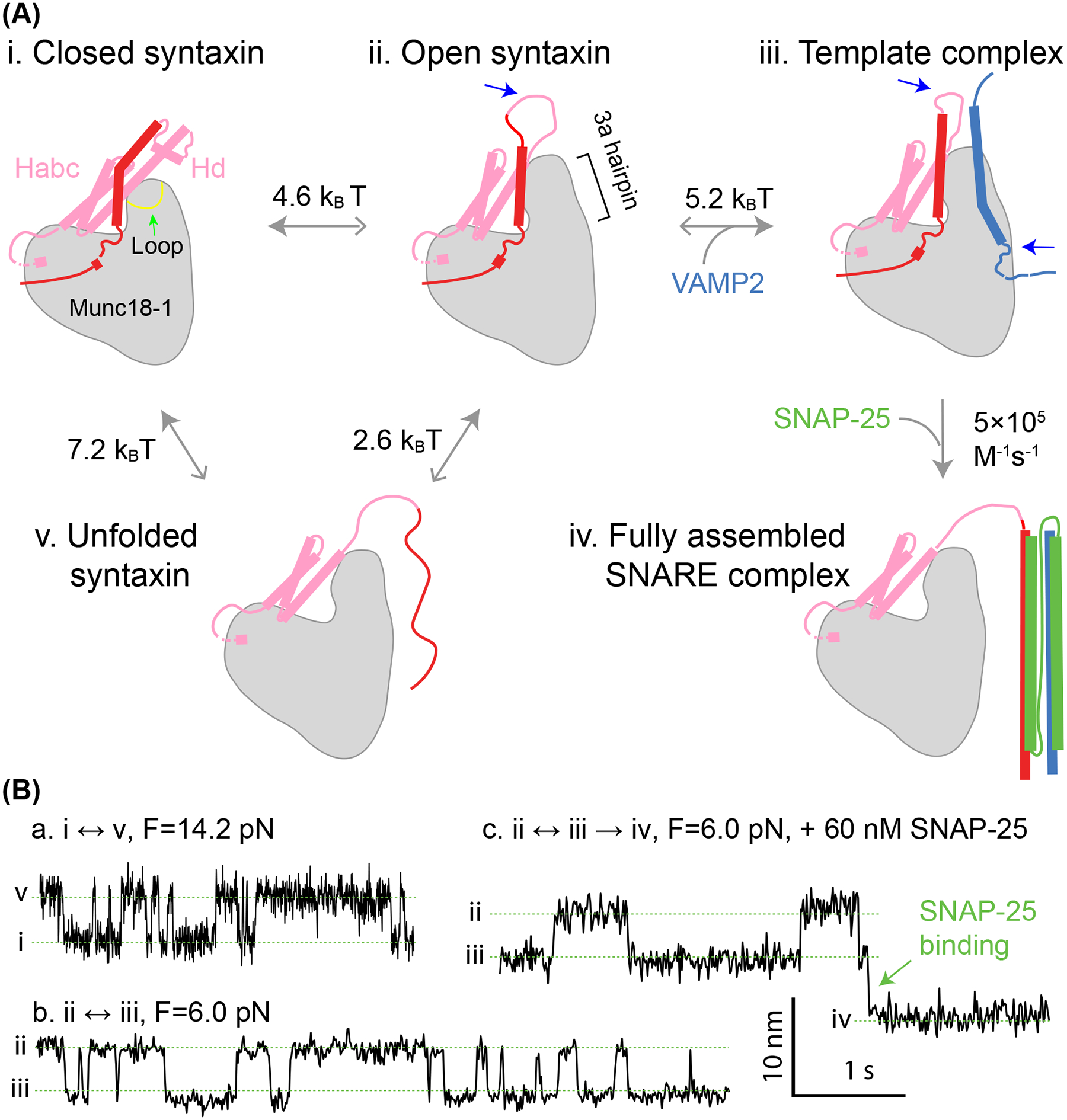
Munc18–1 chaperones SNARE assembly via multiple essential intermediates. (A) The pathway of Munc18–1-chaperoned SNARE assembly. The closed syntaxin (state i) is opened by Munc13–1 (not shown) or mutations to expose the N-terminal region of the SNARE motif in syntaxin (state ii). Syntaxin opening is accompanied by unfurling a loop at the tip of the 3a helical hairpin domain in Munc18–1, as indicated by the green arrow in state i, which extends the 3a domain. Then, the open syntaxin binds to VAMP2 to form the template complex (state iii). The two SNARE binding sites for Munc13–1 (not shown) are indicated by blue arrows. Finally, SNAP-25 associates with the templated SNAREs to form the four-helix bundle (state iv), a process likely accompanied by displacement of Munc18–1 from the SNARE bundle. The free energy to unfold the SNARE motif of syntaxin (state v) has been measured for the closed and open syntaxin. The solid greys arrows point to the states with lower relative free energy. (B) Time-dependent extension trajectories associated with reversible unfolding of the closed syntaxin (a) and the template complex in the absence (b) or presence (c) of SNAP-25 at the indicated force (F).
