Figure 5. α1,2-fucosidase interacts with fucosylated mucosa to enhance adhesion and colonization of Bifidobacterium in the gut.
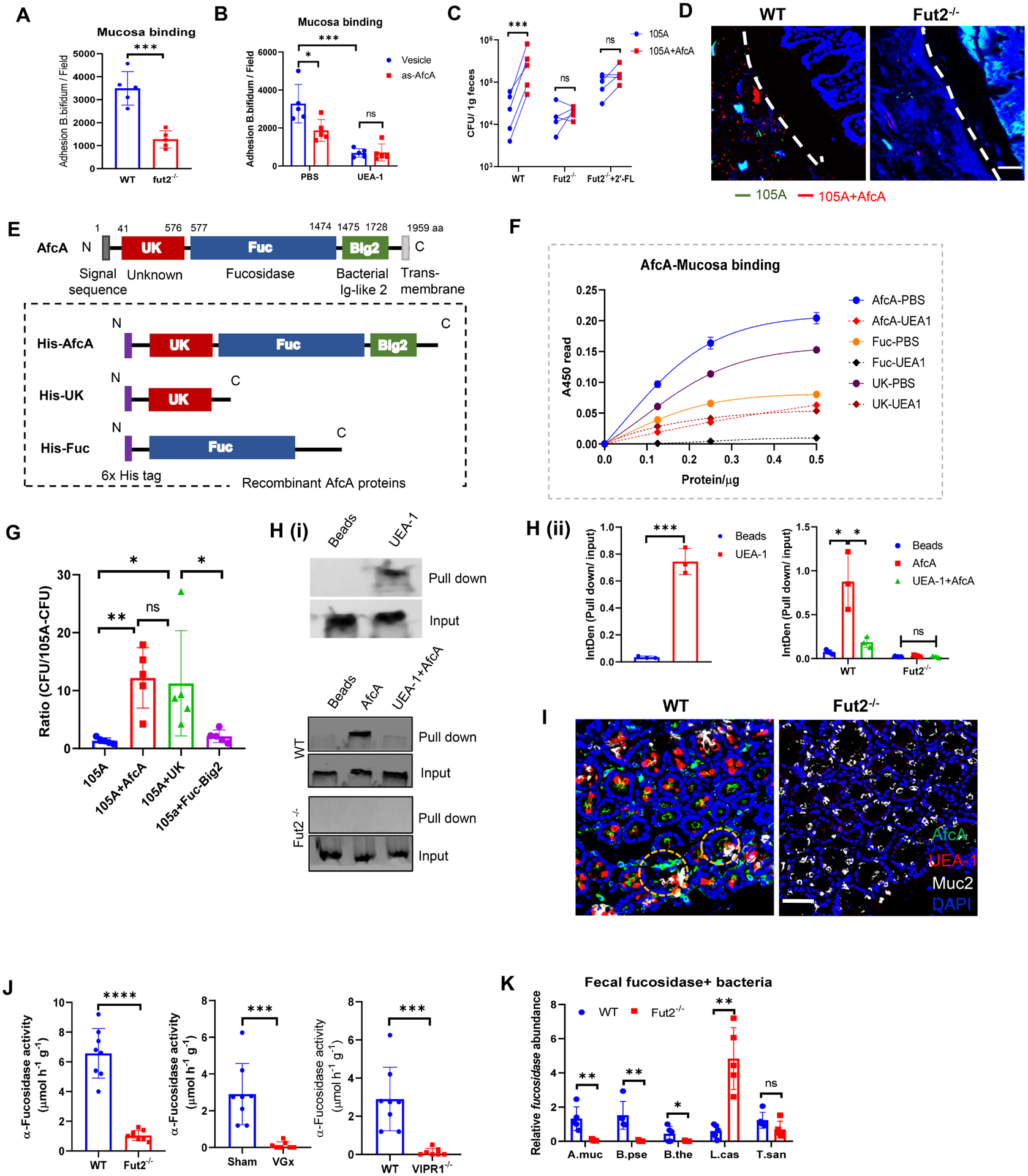
(A) Adhesion of B. bifidum strain to colonic mucosa derived from WT or Fut2−/− mice.
(B) Adhesion of B. bifidum afcA knockdown strain (As-afcA) and control strain to colonic mucosa. UEA-1 was pre-incubated with mucosa for 1h at 37°C before bacteria adding as indicated.
(C) WT mice, Fut2−/− mice or 2’-FL-fed Fut2−/− mice were orally given a 1:1 mixture of B. longum 105A strain (Erythromycin resistant vector-transformed 105A strain (105A)), and afcA-vector-transformed 105A strain (105A+AfcA)). The data show recovery from the feces at 24 h after gavage. (A-C) Data are representative of three independent experiments. Error bars: Mean ± SEM; *p < 0.05; ***p < 0.001.
(D) FISH analysis of B. longum 105-A colonization using a probe for erythromycin (Erm, green) and AfcA (red) within the colonic samples of mice that were gavaged with mixture strains as in C. Scale bar, 50 μm.
(E) (Top) Schematic representation of the primary structure of AfcA from B. bifidum. The grey box at the N terminus indicates a signal peptide. Unknown function domain (UK, 41–576 aa) is depicted as red box. The fucosidase domain (Fuc, 577–1474 aa) responsible for 1,2- fucosidase is depicted as a blue box. The region with Ig-like folds Big2 (1475–1728 aa) is shown by green box; (Bottom) Schematic representation of AfcA-truncated protein expressed as fusions to 6×His tag at the N terminus (purple boxes).
(F) Binding properties of AfcA- full-length or truncated recombinant proteins to mucosa in the absence or presence of UEA-1, as determined by ELISA.
(G) Mice were orally given B. longum 105A strains expressing empty vector (105A), vector with full-length of AfcA (105A+AfcA), the UK domain (105A+UK) or the Fuc+Big2 domain (105A+Fuc−Big2). The data show recovery from the feces at 24 h after infection. Mean ± SEM; n = 5; *p < 0.05; **p < 0.01.
(H) Binding of AfcA recombinant protein with fucosylated Muc2 (i). (Top) Mucosal protein were pulled down by UEA-1 and immunoblotted by anti-Muc2 antibody, a group using streptavidin bead without UEA-1 was set as a blank control (Beads); mucosal protein from WT (Middle) or Fut2−/− mice (Bottom) was pretreated with/without UEA-1 (UEA-1+AfcA or AfcA), pulled down by His-AfcA protein, and then immunoblotted by anti-Muc2 antibody, a group using Ni-NTA bead without his-AfcA was set as blank control (Beads). The qualifications were shown in (ii).
(I) His-AfcA protein binding to murine colonic tissue sections prepared from WT mice or Fut2−/− mice. Total fucosylated conjugates were detected by UEA-1 (red) staining. His-AfcA (green) was detected with anti-His antibody, the goblet cells were indicated by Muc2 (grey) staining. Scale bar, 50 μm.
(J) Measurement of total α-L-fucosidase activity in fecal samples. Fecal supernatant was assayed for cleavage of 4-methylumbelliferyl-fucopyranoside substrate by fluorescence.
(K) Real-time PCR analysis of the relative abundance of α-L-fucosidase producing bacterial strains in the feces of WT mice and Fut2−/− mice. Five representative gut bacteria expressing L-fucosidase were chosen for analysis, including Akkermansia muciniphila (A. muc), Bifidobacterium pseudolongum (B. pse), Bacteroides thetaiotaomicron (B. the), Lactobacillus casei (L. cas) and Turicibacter sanguinis (T. san). Primers target the L-fucosidase genes in the corresponding bacterium were designed for real-time PCR.
Data are from (J-K) at least 2 independent experiments performed. n=5–10, mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns not significant. See also Figure S5.
