Abstract
Background
Quantification of actinium-225 through gamma counter measurements, when there is no secular equilibrium between actinium-225 and its gamma emitting daughters bismuth-213 and/or francium-221, can provide valuable information regarding the possible relocation of recoiled daughters such that related radiotoxicity effects can be evaluated. This study proposes a multiple time-point protocol using the bismuth-213 photopeak with measurements before secular equilibrium between actinium-225 and bismuth-213, and a single time-point protocol using both the francium-221 and bismuth-213 photopeak while assuming secular equilibrium between actinium-225 and francium-221 but not between bismuth-213 and actinium-225.
Results
Good agreement (i.e. 3% accuracy) was obtained when relying on a multiple time-points measurement of bismuth-213 to quantify both actinium-225 and excess of bismuth-213. Following scatter correction, actinium-225 can be accurately quantified using the francium-221 in a single time-point measurement within 3% of accuracy. The analysis performed on the stability data of [225Ac]Ac-DEPA and [225Ac]Ac-DOTA complexes, before secular equilibrium between bismuth-213 and actinium-225 was formed, revealed considerable amounts of unbound bismuth-213 (i.e. more than 90%) after 24 h of the radiolabeling most likely due to the recoiled daughter effect.
Conclusion
Both protocols were able to accurately estimate 225Ac-activities provided the francium-221 energy window was corrected for the down scatter of the higher-energy gamma-emissions by bismuth-213. This could prove beneficial to study the recoiled daughter effect and redistribution of free bismuth-213 by monitoring the accumulation or clearance of bismuth-213 in different tissues during biodistribution studies or in patient samples during clinical studies. On the other hand, the single gamma counter measurement protocol, although required a 30 min waiting time, is more time and cost efficient and therefore more appropriate for standardized quality control procedures of 225Ac-labeled radiopharmaceuticals.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41181-022-00174-z.
Keywords: Actinium-225, Bismuth-213, Francium-221, Gamma counter, Radiopharmaceutical quality control, Recoiled daughter effect
Introduction
Targeted alpha-therapy (TAT) has shown promising results when overcoming resistance to β-emitters in clinical applications (Kratochwil et al. 2014; Ballal et al. 2020). The efficiency of α-particles relies on their short penetration range within tissue (40–100 μm, Allen et al. 2014) and their high linear energy transfer (LET). 225Ac (actinium-225) is considered a promising candidate for TAT and a highly cytotoxic radionuclide due to its relatively long half-life (9.9 days) and the yield of a total of four α particles 221Fr (francium-221): 4.9 min half-life, 6 MeV α-particle; 217At (astatine-217): 32.3 ms half-life, 7 MeV α-particle; 213Bi (bismuth-213): 45.6 min half-life, 6 MeV α-particle; 213Po (polonium-213): 4.2 µs half-life, 8 MeV α-particle) in its decay chain. In addition, two gamma rays are emitted in the decay chain, 218 keV [11.4%] by 221Fr and 440 keV [25.9%] by 213Bi, which can be used for activity measurements. Indeed, most preclinical studies report 225Ac activities which are based on activity measurements of 213Bi or 221Fr while assuming secular equilibrium (SEq) between 225Ac and the measured daughter (Kruijff et al. 2019; Miederer et al. 2004a; Borchardt et al. 2003; Beyer et al. 1990), which means activity measurements based on the gamma emissions by either 221Fr or 213Bi are approximately equal to the activity of 225Ac. However, emission of a high-energy α particle can cause nuclear recoil effect (Kozempel et al. 2018). This recoil energy experienced by the daughter nuclei is sufficient to break the chemical bond between the daughter and the targeting vector (Kruijff et al. 2015), resulting in the so-called recoiled daughter effect (RDE) and causing at least partial release of radioactive daughter nuclei from the original targeting molecule or delivery vehicle (Kruijff et al. 2015). Loss of affinity to the molecular carrier can lead to a redistribution of recoiling radioactive daughters and induce radiation related side effects, such that RDE is often assumed to be the main cause of radiotoxicity for the organs at risk (OAR) and one of the main reasons for limiting the amount of activity of 225Ac-labeled radionuclides administered to patients (Ballal et al. 2020; Kratochwil et al. 2017, 2015; Khreish et al. 2020; Cordier et al. 2010). Redistribution of recoiled daughter radionuclides can also offset the SEq between 225Ac and daughter radionuclides, such that indirect measurement of the activity concentration of 225Ac by measuring the activity concentration of its gamma-ray emitting daughters 221Fr or 213Bi can be biased. Only a few studies (Kruijff et al. 2019; Miederer et al. 2004a, 2008; Poty et al. 2019; Nedrow et al. 2017; Schwartz et al. 2011; Song et al. 2009) have considered measurements before and during SEq between 225Ac and 213Bi, to evaluate the relocation of 213Bi, and in very limited cases also between 225Ac and 221Fr, to evaluate the relocation of 221Fr. Recoiling 213Bi was reported to have affinity to kidney tissue (Kruijff et al. 2019; Song et al. 2009; Drecoll et al. 2009), while 221Fr was associated with uptake in the gastrointestinal tract (Miederer et al. 2004b) and kidneys (Song et al. 2009). However, it should be noted that relocation of 221Fr is generally not considered as relevant because of the very short physical half-life of 221Fr (4.9 min), such that 221Fr is considered as the closest proxy to quantify 225Ac activity concentrations.
In the context of TAT, gamma counting (GC) is a frequently used technique for ex vivo quantification of 225Ac-labeled radiopharmaceuticals (Castillo Seoane et al. 2020), especially since in vivo nuclear imaging techniques, such as single-photon emission computed tomography (SPECT), have limited potential to allow accurate quantitative imaging of 225Ac concentrations in tissues because of the low branching ratio of gamma-emissions in the decay chain and the low administered activities. Therefore, other measurement techniques, such as GC, represent an asset for dosimetry and radiotoxicity estimates, both preclinically and clinically. In a preclinical setting, GC allows biodistribution studies of 225Ac-labeled radiopharmaceuticals to estimate the absorbed doses by tumoral and healthy tissue. In addition, GC provides valuable information regarding the possible relocation of recoiled daughters, which is of interest to evaluate related radiotoxicity effects. In a clinical setting, GC measurements of blood and urine samples can be considered to determine plasma and renal clearance of radiopharmaceuticals, which in turn can be used for compartmental modeling of pharmacokinetics to estimate the radiation burden to OAR (Siegel et al. 1999).
In addition, GC measurements play an important role in the quality control (QC) of 225Ac-labeled radiopharmaceuticals to confirm sufficiently high radiochemical yield (RCY) before administration to patients. For this purpose, the activity distribution on an instant thin-layer liquid chromatography (iTLC) strip is analyzed using GC measurements to estimate the fraction of bound and unbound 225Ac and its daughter 213Bi. These GC measurements are usually based on the gamma emissions by 221Fr, which is at SEq with 225Ac within approximately six half-lives of 221Fr (~ 30 min) (Hooijman et al. 2021).
However, limited research has been done on GC measurements to quantify 225Ac activity when there is no SEq between 225Ac and its gamma emitting daughters 213Bi and/or 221Fr. Generally, GC measurements are delayed ensuring sufficient time for 213Bi to reach SEq with 225Ac, and to provide an unbiased estimation of the 225Ac-activity. The limitation of this approach is that, once in SEq, any additional 213Bi activity which was originally present in the measured sample before the start of the GC measurements cannot be traced back. Nonetheless, there is a growing interest to quantify 213Bi activity which is not related to the parent–daughter decay of 225Ac, but that is generated by the potential RDE and relocation of 213Bi. Therefore, the aim of this study is to develop and validate GC protocols to accurately quantify both 225Ac activity and a potential accumulation or clearance of 213Bi activity compared to SEq between 225Ac and 213Bi. We proposed (1) a multiple time-point protocol using the 213Bi photopeak with measurements before SEq between 225Ac and 213Bi, and (2) a single time-point protocol using both the 221Fr and 213Bi photopeaks, while assuming SEq between 225Ac and 221Fr but not between 213Bi and 225Ac. Using these two protocols, we evaluated the amount of unbound 213Bi for two different chelators [225Ac]Ac-DEPA(7-[2-(bis-carboxymethyl-amino)-ethyl]-4,10-bis-carboxymethyl-1,4,7,10- tetraazacyclododec-1-yl-acetic acid) and [225Ac]Ac-DOTA (1,4,7, 10-Tetraazacyclododecane-1, 4,7,10-tetracetic acid) using iTLC strips after incubation in human serum to evaluate stability and RDE as part of QC tests after radiolabeling.
Materials and methods
Radioisotopes and preparation of [225Ac]-labeled constructs
225Ac samples were obtained from recurrent (trimestral, 6 MBq) milkings of an in-house 229Th stock obtained from a processed 229Th capsule as described by Boden et al. (2017). Milkings were performed with the aid of a tandem system of extraction chromatography columns using TEVA and DGA columns obtained from TrisKem, France. A first batch of 225Ac was used to evaluate the GC linearity and the effect of the sample volume variation on the GC detection efficiency (for details on the sample volume variations see Additional file 1).
Additionally, ± 1.3 MBq of 225Ac was used to elute 1.19 MBq of 213Bi (using 0.1 M HCl/ 0.1 M NaI) from an 225Ac/213Bi generator loaded with an AG MP-50 cation exchange resin (Ahenkorah et al. 2021). The eluate 213BiI4−/213BiI52− was used to prepare two samples, one containing 225Ac in SEq with 213Bi and 221Fr for the GC calibration, and one containing pure 213Bi to determine the photon scatter contribution of 213Bi gamma-emissions in the photopeak window of 221Fr.
Two other solutions of 225Ac doped with additional 213Bi were prepared to create a mixture of 225Ac in SEq with 213Bi and additional, pure 213Bi, to simulate non-equilibrium conditions between 225Ac and 213Bi. As such, the total activity of 213Bi was given by:
| 1 |
where is the activity of 213Bi in SEq with 225Ac. This was done by starting from a solution (~ 0.5 mL) of 225Ac in SEq with its decay progeny and adding 0.2 mL (and 0.3 mL) of the stock solution of pure 213Bi to 0.3 mL (and 0.2 mL) of the solution of 225Ac to obtain two samples with additional 213Bi activity (total sample volume ~ 0.5 mL).
225Ac-constructs were synthesized by adding [225Ac]Ac(NO3)3 (1–2 MBq, 100 μL, 0.05 M) to a Tris–HCl buffer (0.37 M, pH 8.5, chelex treated) solution containing DEPA or DOTA (50 µM) and reacting in a glass vial for 60 min at 95 °C. 225Ac-constructs were purified using a C18 Plus Sep-Pak cartridge (Waters Co., Milford, MA, USA) by loading the reaction mixture, rinsing with water (5 mL) to remove unreacted [225Ac]Ac(NO3)3, and eluting the purified complex with absolute ethanol (0.5 mL) as described by Cassells et al. (2021). After purification, radiolabeled aliquots were applied immediately to the test media.
The radioactive composition of all reference samples (i.e. stock solutions) were determined via high-resolution gamma spectrometry analysis using a high-purity germanium (HPGe) detector (Mirion-Canberra, Meriden, USA) calibrated for photon energy and detection efficiency. 225Ac quantities were determined indirectly via measurements of 221Fr and 213Bi photopeaks once SEq was established. 213Bi quantities were determined via measurement of a pure 213Bi sample as fast as possible after elution. The content mass of the sample was verified gravimetrically and the exact volumes of the radioactive samples in each test tube were determined by weighing each tube before and after filling with an analytical balance (model ABP-200-4M; KERN & SOHN GmbH, Germany). The reference activity in each test tube was determined from the volume of each sample and the activity concentration of the reference stock solution. The relative statistical uncertainty of the reference activity concentration of each of the calibration stock solutions was always within 3.2% at 95% CI (coverage factor k = 2).
Gamma counter measurements
Gamma counter activity measurements were done in a 2480 Wizard2 gamma counter (Perkin Elmer, Waltham, MA, USA) using a measurement protocol optimized to limit the overall measurement uncertainty (cfr. details in the Additional file 1). This gamma counter consists of a single 75-mm-diameter and 80-mm-high NaI(Tl) well-type detector. Each radioactive sample was measured for 60 s in a standard tube (5 mL plastic vials of 75 mm height and 12 mm diameter), using a fixed energy window of 175–282 keV () and 378–520 keV () corresponding to the main photopeaks of 221Fr (218 keV) and 213Bi (440 keV), respectively. The measurement in each EW setting was automatically corrected for dead time, background, and measurement time and expressed as counts per minute (CPM).
First, the linear range of GC measurements for the two energy windows was determined, together with the GC calibration factor (CF). For the impact of different sample volumes on the detection efficiency, we refer to the Additional file 1.
Assuming SEq between 225Ac and 213Bi (and thus 221Fr), the calibration factors for () and () were determined from GC measurements as:
| 2 |
| 3 |
If there is SEq between 225Ac and 221Fr but not between 225Ac and 213Bi, we want to have the CPM measured with to be independent of the 213Bi activity. Therefore, we needed to correct for the down scatter of the higher high-energy gamma rays of 213Bi into the (Fig. 1). Hence, the scatter contribution of 213Bi to () was determined as function of by performing GC measurements using both and for a pure 213Bi sample which was measured for more than 5 h to cover different values. As such GC measurements using the can be corrected for the 213Bi down scatter by subtracting the activity-dependent scatter contribution of 213Bi to the using the following expression:
| 4 |
Fig. 1.
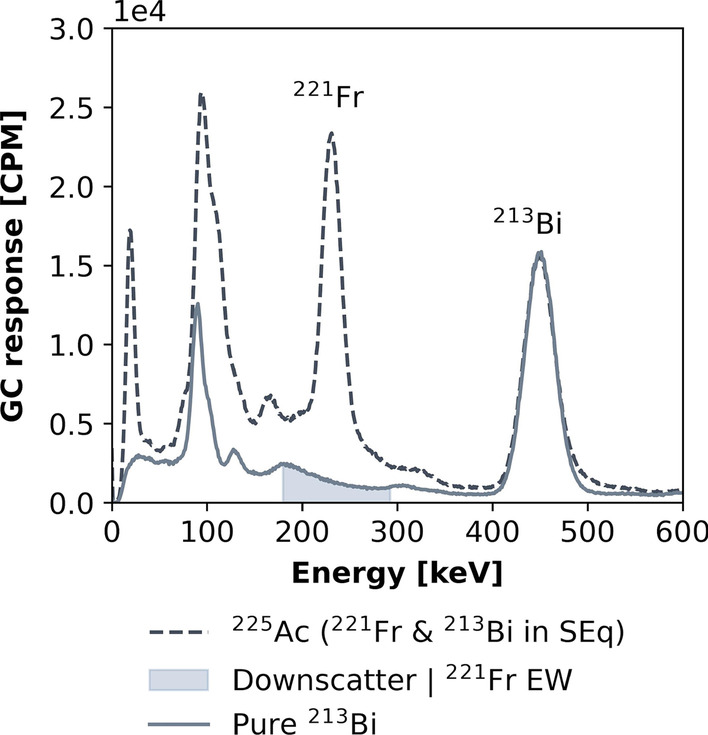
GC photon energy spectrum measured for 225Ac, in SEq with both 221Fr and 213Bi, and for pure 213Bi
This resulted in a calibration factor for the scatter-corrected to accurately estimate 225Ac activities independent of 213Bi activities:
| 5 |
Multiple time-point GC measurements to quantify 225Ac doped with 213Bi
Immediately after preparation of the two samples with an additional amount of 213Bi activity (compared to 213Bi activity in SEq with 225Ac), GC measurements were performed sequentially, at intervals of 5 min, for a total duration of 5–6 h after preparation, using the and corresponding . Given , the initial activity of 213Bi in SEq with 225Ac, and , the additional amount of 213Bi activity at the start of the measurement (t = 0), the total activity of 213Bi at time t is given by (see Eqs. 1, 2):
| 6 |
with the GC measurement at time t using and with and the physical decay constants for 225Ac and 213Bi respectively. Therefore, GC measurements using were analyzed as a function of time t after start of the measurement and used to determine the initial 213Bi activity in SEq with 225Ac and the additional amount of 213Bi at the start of the GC measurement. This was done by non-linear least squares fitting (GraphPad Prism version 9.1.0) of a double exponential function to the GC measurements at different time-points with the physical decay constants fixed.
Single time-point GC measurements to quantify 225Ac doped with 213Bi
In the second approach, a single time-point GC measurement using both and was considered, which theoretically should allow an estimation of both 225Ac activity in SEq with 213Bi and an additional amount of 213Bi activity. This approach assumes one single GC measurement after SEq is reached between 225Ac and 221Fr (after ~ 30 min), but as soon as possible thereafter, to ensure that any 213Bi present in the sample has not yet reached SEq with 225Ac. This way, one can still differentiate between the amount of 213Bi activity in SEq with 225Ac and the additional amount of 213Bi activity. If we assume the single time-point GC measurement at time t after sample preparation, the additional amount of 213Bi activity at the start of the measurement () can be estimated using the once the initial activity of 225Ac () at the start of the measurement is known:
| 7 |
with the GC measurement at time t using and with and the physical decay constant for 225Ac and 213Bi respectively. In turn, the initial activity of 225Ac () can be estimated using the either from:
| 8 |
with the GC measurement at time t using without scatter correction (see Eq. 3), or from:
| 9 |
with the GC measurement at time t using with scatter correction (see Eq. 5). In both Eq. 8 and Eq. 9, represents the physical decay constant for 225Ac.
Both Eqs. 8 and 9 were considered to estimate the initial activity of 225Ac using .
Quantification of the bound fraction of 225Ac and 213Bi during radiopharmaceutical QC of [225Ac]Ac-DEPA and [225Ac]Ac-DOTA complexes
As a direct application, we performed GC measurements at multiple time-points during the stability test of two constructs, [225Ac]Ac-DEPA and [225Ac]Ac-DOTA, as part of radiopharmaceutical QC. For this stability test, two ethanolic solutions of 50 μL, each containing one of the constructs, were added immediately after purification to 450 μL of human serum and incubated at 37 °C. Samples were collected at 15 min and 24 h post reaction. The RCY of each reaction mixture was determined by instant thin-layer liquid chromatography (iTLC-SG, Varian, Diegem, Belgium) with an elution chamber using acetonitrile/water (75%/25% v/v) such that bound 225Ac and bound daughter radionuclides will migrate with the solvent front to the upper part of the iTLC strip, while unbound radionuclides will remain at the lower part where the mixture was originally spotted (Fig. 2) (Cassells et al. 2021). Once the solvent front reached the 1-cm mark of the iTLC strip at time ti (cfr solvent front Fig. 2), the iTLC strip is removed from the mobile phase and cut in half. Immediately after, the activity of the upper and lower parts of the iTLC strip were measured with GC using both and with the previously described energy window settings.
Fig. 2.
Schematic representation of the migration of radiolabeled compounds in the iTLC strip. Upon exposure of the strip to the mobile phase, unbound radionuclides will remain in the spotting site, whereas bound radionuclides will move with the solvent front to the upper part of the iTLC strip
The 225Ac activity was estimated for each part of the iTLC strip using a single time-point GC measurement after more than 5 h to ensure SEq between 225Ac and 213Bi such that the 225Ac activity could be estimated from the CPM data acquired with and extrapolated to the start of the iTLC elution at t0.
In addition, a single time-point measurement was performed at 30 min after starting the iTLC elution to ensure SEq between 225Ac and 221Fr such that the 225Ac activity could be estimated from the CPM data acquired with with (Eq. 9) and without scatter correction (Eq. 8). Next, the increase (or decrease) in 213Bi activities compared to SEq (dependent on the part of the strip) was estimated from the CPM acquired with . In case of an increase of 213Bi, the 213Bi activity at the start of the iTLC elution (t0) was estimated using Eq. 7.
For the upper part of the iTLC strips obtained after 24 h of incubation in human serum, multiple time-point measurements were performed before SEq between 225Ac and 213Bi (> 5 h) while only the CPM data acquired with the were used for the analysis. In case of increased or reduced 213Bi activity compared to SEq with 225Ac, fitting of a double exponential function (i.e. Eq. 6) was performed by non-linear least squares fitting (GraphPad Prism version 9.1.0) to the multiple GC measurements at different time-points. For both 225Ac and 213Bi, the bound fraction was determined as the ratio of the activity of either radionuclide measured for the upper part of the iTLC over the sum of activities measured for both the upper and the lower part of the iTLC.
Results
225Ac/213Bi samples and [225Ac]-labeled constructs
Table 1 gives an overview of the different samples for GC measurements and the activity at the start of the measurements. To evaluate the GC linearity a total of 19 test samples were prepared from sample 1. The samples were prepared with equal volume (0.5 mL), to avoid a volume effect on sensitivity, and with varying activities ranging from 1 Bq up to 500 kBq of 225Ac in SEq with 221Fr and 213Bi.
Table 1.
Overview of the different samples for GC measurements and the activity at the start of the measurements
| Sample | 225Ac (in SEq with 213Bi) | Added 213Bi | Experiment |
|---|---|---|---|
| 1 | 1.5 MBq (total) | 0 kBq | GC calibration |
| 2 | 0 kBq | 117 kBq | Quantifying down scatter of higher-energy gamma emissions by 213Bi in |
| 3 | 94 kBq | 55 kBq | Simulating non-SEq conditions between 225Ac and 213Bi |
| 4 | 116 kBq | 32 kBq | |
| 5 | 1–2 MBq | 0 kBq | Radiolabeling of [225Ac]Ac-DOTA and incubation in human serum for 15 min and 24 h |
| 6 | 1–2 MBq | 0 kBq | Radiolabeling of [225Ac]Ac- DEPA and incubation in human serum for 15 min and 24 h |
Specifications of the samples used for determining the linear range of GC measurements and evaluating the impact of different sample volumes on GC measurements is given in the Additional file 1
Gamma counter measurements
For both EW setting for 221Fr and 213Bi, the GC response expressed as count rate (CPM) was measured as a function of the different activities obtained from sample 1 which was cross-calibrated with a standard HPGe detector (see Fig. 3). A non-linearity of more than 3% in the GC response was observed for activities above 150 kBq, resulting in a CPM underestimation of up to 17% and 25% for activities around 0.5 MBq when using the and the respectively. Therefore, GC response was considered linear up to 150 kBq 221Fr and 213Bi, in SEq with 225Ac. For this linear range, CFs were determined by linear regression, resulting in GC CFs for the EWs of 213Bi () and 221Fr () of 6.1E+06 CPM/MBq and 7.4E+06 CPM/MBq, respectively. The CPMs obtained with GC using the () are shown as a function of different CPM measured with () for different 213Bi activities obtained from a cross-calibration with the HPGe detector (see Fig. 4). Results indicated a clear, linear relationship between and (linear regression, slope = 0.205 ± 0.001, R2 > 0.99) which can be used to estimate the scatter contribution of 213Bi to GC measurements in the . This scatter fraction can be subtracted from , and 225Ac activities can be accurately estimated even when there is no SEq between 225Ac and 213Bi (Eq. 9). The corresponding GC calibration factor for the , when a correction for 213Bi photon down scatter is applied, was determined as 6.1E+06 CPM/MBq.
Fig. 3.

GC response for 225Ac in SEq with 221Fr and 213Bi, measured with both EWs of 221Fr and 213Bi. Error bars represent the standard deviation provided by the GC software
Fig. 4.
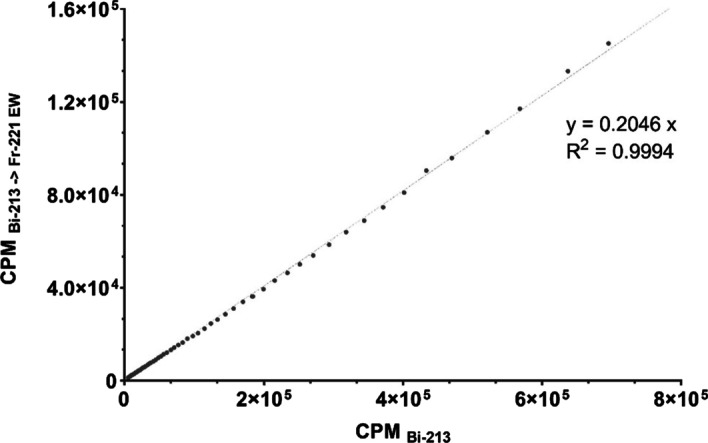
Count rate of the down scatter of gamma emissions by 213Bi in the () for different count rates of 213Bi measured with the ()
Multiple time-point GC measurements to quantify 225Ac doped with 213Bi
For the multiple time-point approach, activities measured by the gamma counter using are plotted as a function of the measurement time for the sample 3 (see Table 1) containing 213Bi activity in SEq with 225Ac and an additional amount of 213Bi activity (see Fig. 5). Based on a bi-exponential fit with fixed physical decay constants for 225Ac and 213Bi (cfr Eq. 6), 225Ac and 213Bi activities were estimated (see Table 2), both with a relative percentage difference lower than 3% compared to the reference activities determined with the HPGe detector.
Fig. 5.

213Bi activities of sample 3 (see Table 1) containing 213Bi in SEq with 225Ac and an added amount of 213Bi. GC measurements were performed at different time-points before and after SEq between 213Bi and 225Ac using the . Error bars represent the standard deviation provided by the GC software
Table 2.
Multiple and single time-point (at 30 min) estimation of the 225Ac and 213Bi activity for sample 3 and 4 containing 213Bi in SEq with 225Ac and an additional amount of 213Bi
| CPM | 213Bi (SEq with 225Ac) | Added 213Bi | |||
|---|---|---|---|---|---|
| kBq | %diff | kBq | %diff | ||
| Sample 3 | |||||
| Multiple time-points | 93.7 | < 3 | 54.9 | < 3 | |
| Single time-point at 30 min | , | 101.8 | 9 | 47.0 | − 15 |
| Single time-point at 30 min (scatter corrected) | , | 93.4 | < 3 | 55.3 | < 3 |
| Sample 4 | |||||
| Multiple time-points | 116.2 | < 3 | 32.9 | < 3 | |
| Single time-point at 30 min | , | 122.9 | 6 | 28.4 | − 11 |
| Single time-point at 30 min (scatter corrected) | , | 116.7 | < 3 | 32.8 | < 3 |
Corresponding CPM approaches are indicated and the relative difference (%diff) compared to the reference activities in both samples
Single time-point GC measurements to quantify 225Ac doped with 213Bi
For the single time-point approach, 225Ac activities measured with with and without scatter correction are plotted as function of time for the sample 3 containing 225Ac in SEq with 213Bi and additional 213Bi activity (Fig. 6A). This measurement was started after SEq was reached between 221Fr and 225Ac (~ 30 min) such that 225Ac activity could be estimated from the CPMs measured within . When no scatter correction was applied, 225Ac activities showed an overestimation due to the additional down scatter of gamma-emissions by the added 213Bi activity. The overestimation gradually decreased because of the decay of this additional 213Bi till SEq was again reached between 225Ac and 213Bi (Fig. 6B). Applying a correction for down scatter of 213Bi gamma emissions in the reduces this overestimation of 225Ac activities. For a single time-point GC measurement at 30 min, omission of the scatter correction resulted in an overestimation of 6 to 9% of the 225Ac activity while the estimated activity was within 3% of the reference value when scatter correction was applied. When using the estimated 225Ac activity to determine the additional 213Bi activity, this resulted in an underestimation of 11 to 15% when no scatter correction was applied and while it was within 3% of the reference activity after applying a correction for down scatter (Table 2).
Fig. 6.
A 225Ac activities measured with with and without scatter correction are plotted as function of measurement time for the sample 3 (see Table 1) containing 225Ac and additional 213Bi activity. Error bars represent the standard deviation provided by the GC software. B Relative bias (percentage relative difference) of 225Ac activity estimated using as function of the added 213Bi activity (compared to SEq between 225Ac and 213Bi) when no correction for the additional down scatter of 213Bi gamma-emissions in the is applied
Quantification of 225Ac and 213Bi during radiopharmaceutical QC of [225Ac]Ac-DEPA and [225Ac]Ac-DOTA complexes
An overview of the bound fraction of [225Ac]Ac-DEPA/DOTA after a 15 min and 24 h incubation in human serum is given in Table 3. These estimates are based on single time-point GC measurement protocols.
Table 3.
Bound fraction of 225Ac (activity upper part iTLC/activity iTLC) to [225Ac]Ac-DEPA/DOTA complexes for samples 5 and 6 (see Table 1) after 15 min and 24 h incubation in human serum
| EW | CPM | [225Ac]Ac-DOTA | [225Ac]Ac-DEPA | ||
|---|---|---|---|---|---|
| 15 min | 24 h | 15 min | 24 h | ||
| (at 5 h) | 88% | 91% | 96% | 96% | |
| (at 30 min, without scatter correction) | 84% | 78% | 88% | 83% | |
| , (at 30 min, with scatter correction) | , , | 89% | 90% | 95% | 93% |
225Ac activities were determined with a single time-point GC measurement using at 5 h, to ensure SEq between 213Bi and 225Ac, and using with and without scatter correction at 30 min, to ensure SEq between 221Fr and 225Ac
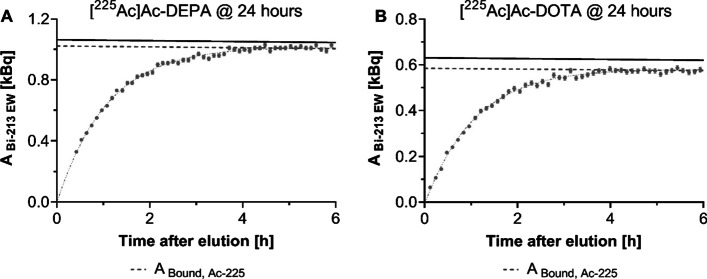
Multiple time-point GC measurements, performed of the upper part of iTLC strip for the 24 h incubation time in human serum using (see Fig. 7), showed an ingrowth of 213Bi for both [225Ac]Ac-DOTA and [225Ac]Ac-DEPA until SEq was again restored between 225Ac and 213Bi after 5 h (i.e. 213Bi reached ~ 99% of 225Ac activity). These findings correspond to a lower fraction of bound 213Bi compared to the fraction of bound 225Ac.
Fig. 7.
Multiple time-point GC measurements were performed of the upper part of iTLC strip using before SEq was reached between 213Bi and 225Ac (< 5 h), to determine the amount of bound 213Bi after 24 h of incubation in human serum of [225Ac]Ac-DEPA (A) and [225Ac]Ac-DOTA (B). The solid line represents the total amount of 225Ac (from GC of both parts of the iTLC strips) and the dotted line the amount of bound 225Ac. Error bars represent the standard deviation provided by the GC software
For [225Ac]Ac-DOTA, fitting of the non-linear least squares fitting of a double exponential (i.e. Eq. 6) function to the multiple GC measurements at different time-points estimated the initial 213Bi activity in the upper part of the iTLC strip as zero, within a 95% confidence interval (0.01 ± 0.02 kBq), meaning that no bound 213Bi was present in these samples after 24 h of incubation in human serum.
Discussion
This study focused on GC measurements to quantify 225Ac activity when SEq is not guaranteed or not yet reached between 225Ac and its daughter radionuclide 213Bi. Before performing our experiments, we first determined the linear range and calibration factor of the GC system and ensured that all activities for this study were within this range to guarantee optimal quantitative performance. In addition, samples were prepared to have an equal volume of 0.5 mL to avoid a volume effect on the detection efficiency. As GC measurements of 225Ac-activity rely on the gamma emissions by daughter radionuclides 213Bi and 221Fr, this indirect approach requires SEq between 225Ac on the one hand and 213Bi and/or 221Fr on the other hand, especially if only one time-point is measured. When using an EW for 213Bi, one should wait for more than 5 h to ensure that 213Bi and 225Ac activities are in agreement (~ 99%) and bias on 225Ac activity measurements is avoided as much as possible. While this long waiting time can prove to be challenging from a practical point of view, it also prevents the quantification of 213Bi activities before SEq with 225Ac. This information is however needed to monitor the relocation of free 213Bi, released from the molecular vector, from the tumor site or tissues showing target expression to organs involved in excretory pathway of 213Bi. A straightforward approach to avoid the need for SEq between 225Ac and 213Bi when using the EW of 213Bi for 225Ac quantification, is a multiple GC measurement protocol where activities are measured at different time points before and/or during SEq between 225Ac and 213Bi. As the approach does not require SEq, neither between 225Ac and 213Bi nor between 225Ac and 221Fr, GC measurements can be initiated as soon as samples are available to ensure that the highest activity of 213Bi can be measured. This can be very useful for biodistribution studies where the 213Bi activity in the different organs is not known in advance and sensitivity to pick up even low levels of 213Bi activities should be maximized. For our experiments, we performed measurements every 5 min to demonstrate feasibility but in practice, the optimal timing for the measurements will depend on the number of samples which need to be counted and the availability of the GC system. However, we would advise to maximize the number of measurements to reduce the impact of noise, since 213Bi activities are expected to be rather low in preclinical biodistribution and radiotoxicity studies on rodents (Kruijff et al. 2019; Miederer et al. 2004a, 2008; Poty et al. 2019; Nedrow et al. 2017; Schwartz et al. 2011). In addition, this approach using multiple GC measurements could also be considered for monitoring whether 221Fr activities are different from the activities expected for SEq with 225Ac. However, non-SEq between 221Fr and 225Ac is generally considered irrelevant and challenging to assess, due to its relatively short physical half-life of only a few minutes. Moreover, because 221Fr is the first gamma-emitting daughter in the decay chain of 225Ac, with this short half-life, it is generally considered as the closest proxy daughter to determine the presence/location and activity of 225Ac.
Therefore, we suggested a single GC measurement to determine the 225Ac activity using the EW of 221Fr. This can be done once SEq is established between 225Ac and 221Fr around 30 min after mixed 225Ac/213Bi samples have been synthetized as was previously reported (Hooijman et al. 2021; Pretze et al. 2021). However, we noticed that 225Ac activity estimated with the EW of 221Fr is biased when the activity of 213Bi does not correspond with the expected activity in case of SEq with 225Ac. This problem can be addressed by using a HPGe detector due to its superior energy resolution which provides a more definite isotopic identification for low-energy emitters than NaI(Tl) detectors (Perez-Andujar and Pibida 2004). However, the purpose of this study was to optimize the GC protocols because these detectors are much more accessible than HPGe detectors. Moreover, commercial GC systems allow automatic measurements of many samples, making it a very suitable technique for the multiple time point protocols that were used for this study. Therefore, we advise to estimate the down scatter of the higher-energy gamma rays by 213Bi into the EW of 221Fr when using GC protocols. This way, the count rate in the EW of 221Fr can be corrected, such that 225Ac-estimates using the EW of 221Fr are unbiased and independent of the 213Bi activity present in the sample or mixture. Once 225Ac activity has been determined, the remaining 213Bi activity can be estimated using the EW of 213Bi. However, this single GC measurement approach requires a waiting time of around 30 min which reduces the sensitivity for measuring low amounts of 213Bi compared to a GC measurement protocol using multiple time points. On the other hand, a single GC measurement protocol can be considered when higher levels of 213Bi activities are anticipated, like for example during the synthesis of 225Ac-labeled radiopharmaceuticals, or when standardized QC procedures need to be balanced between unbiased activity estimates and short measurement times. A single GC measurement at 2 h after radiolabeling was already suggested as ideal time point for GC measurements to balance the need for a fast release and accurate assessment of the radiochemical yield (Kelly et al. 2021; Eryilmaz and Kilbas 2021). However, one could argue that 2 h waiting time will delay administration to patients, while the bound fraction of both 213Bi and 225Ac will be reduced to the RDE. Therefore, a GC measurement protocol at 30 min to indirectly quantify 225Ac activity by measuring 221Fr once SEq is reached between 225Ac and 221Fr, can be a valid alternative, provided that a correction is applied for the down scatter of the higher-energy gamma emissions by 213Bi.
For our study, we used both single and multiple time-point GC measurements to evaluate the stability of [225Ac]Ac-DEPA and [225Ac]Ac-DOTA constructs in human serum to simulate an in vivo situation and challenge the radiocomplexes to test their stability (e.g. tranchelation by metalloproteins). After 24 h of incubation in human serum, negligible amount of bound 213Bi were observed for both constructs based on the GC measurements of the upper part of the iTLC strips just after elution (see Fig. 7). Following the GC measurements, 213Bi activity gradually recovered till SEq was reached with 225Ac, and 213Bi activity was again representative for the fraction of bound 225Ac. These findings showed that, after 24 h incubation in human serum, 213Bi was not bound to the chelator anymore and was most likely released due to the RDE, while the fraction of bound 225Ac remained very high (Kratochwil et al. 2016) and very stable for both constructs (see Table 3). To reduce potential radiotoxicity caused by the RDE, diethylenetriamine pentaacetic acid (DTPA, hydrophilic chelate) can be added to the final formulation buffer for complexation of free 225Ac and recoiled daughters before administration and combined with diuretic drugs to increase renal excretion (Kratochwil et al. 2016). However, once administered, the 225Ac-labeled compound is being added to a diluted medium, such as blood pool, and 213Bi will still be released and cause additional radiotoxicity because of RDE. This can justify, to some extent, the high uptake of free 213Bi in healthy (i.e. non-targeted) organs (e.g. kidneys Kruijff et al. 2019; Song et al. 2009; Drecoll et al. 2009) early after injection. Ideally, this risk of potential redistribution and additional radiotoxicity should be minimized by using more stable targeting systems with high radiolabeling yields capable of retaining part of the daughter nuclides or strategies to retain radionuclides in the tumor cells/tissues (Kruijff et al. 2019; Robertson et al. 2018).
In terms of clinical translation, one could consider using the proposed GC protocols to quantify activity levels of 225Ac and 213Bi in blood and urine samples of patients undergoing 225Ac -TAT. This way, renal, bone marrow, urinary bladder wall, or gastrointestinal toxicity can be estimated and whole-body clearance can be monitored in a more patient-specific manner, contrary to the more empirically dose estimates to OARs based on the retrospective evaluation of radiotoxicity and treatment response in groups of patients (Siegel et al. 1999). Moreover, accurate GC measurements of the 225Ac and 213Bi activity levels in patient samples could be combined with compartmental models to unravel the biokinetics of 225Ac-pharmaceuticals and recoiling daughters for a better dosimetry (Hooijman et al. 2021), such to allow better prediction of radiotoxicity and treatment efficacy in patients.
The main limitation of this study is the limited number of experiments because of the limited availability of 225Ac. Therefore, the proposed GC protocols should be further validated and especially the time schedule of the multiple GC measurements for each specific application.
Conclusions
For this study, we evaluated two GC measurement protocols for quantifying 225Ac-activity when no SEq is guaranteed between 225Ac and its daughter radionuclide 213Bi. A first protocol performed multiple measurements using only the 213Bi energy window and requires no secular equilibrium between 225Ac and its gamma emitting daughter radionuclides. For the second protocol, SEq was required between 225Ac and 221Fr corresponding to a waiting time of around 30 min but only one measurement was needed using both the 213Bi and 221Fr energy window. Both protocols were able to accurately estimate 225Ac-activities provided the 221Fr energy window was corrected for the down scatter of the higher-energy gamma-emissions by 213Bi. In addition, these two protocols were able to quantify 213Bi-activities which cannot be attributed to the parent-daughter decay of 225Ac. From this perspective, a multiple GC measurement protocol could prove more sensitive to pick up low levels of 213Bi since it doesn’t require SEq and allows measurements as soon as samples are available. This could prove beneficial to study the recoil daughter effect and redistribution of free 213Bi by monitoring the accumulation or clearance of 213Bi in different tissues during biodistribution studies or in patient samples during clinical studies. On the other hand, the single GC measurement protocol, although required a 30 min waiting time, is more time and cost efficient and therefore more appropriate for standardized QC procedures of 225Ac-labeled radiopharmaceuticals.
Supplementary Information
Additional file 1: Fig. S1. Relative counting efficiency of the GC as function of sample volume for the quantification of 225Ac using the 213Bi and 221Fr EW. Design and results of an additional experiment to study the effect of sample volume on the relative GC detector efficiency.
Acknowledgements
We thank Stephan Heinitz and Hongshan Zhu for their technical assistance during the preparation of the 225Ac/213Bi generator.
Abbreviations
- CF
Calibration factor
- CPM
Counts per minute
- DOTA
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetracetic acid
- DEPA
7-[2-(Bis-carboxymethyl-amino)-ethyl]-4,10-bis-carboxymethyl-1,4,7,10-tetraazacyclododec-1-yl-acetic acid
- DTPA
Diethylenetriamine pentaacetic acid
- EW
Energy window
- GC
Gamma counter
- iTLC
Instant thin-layer liquid chromatography
- LET
Linear energy transfer
- QC
Quality control
- RCY
Radiochemical yield
- RDE
Recoil daughter effect
- SEq
Secular equilibrium
- SPECT
Single photon emission computed tomography
- TAC
Time–activity curves
- TAT
Targeted alpha-particle therapy
Author contributions
DCS: experimental design, experimental setup, data analysis, discuss data analytics, draft manuscript. MDSH: methodology, discuss data analytics, draft manuscript. SA: experimental design, experimental setup, radiopharmaceutical radiolabeling, discuss data analytics, writing-review. CSV: experimental design, discuss data analytics, methodology, writing-review. MO: radiopharmaceutical radiolabeling, discuss data analytics, review. LS: methodology, discuss data analytics, writing-review. MK: methodology, discuss data analytics, draft manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahenkorah S, Cassells I, Deroose CM, Cardinaels T, Burgoyne AR, Bormans G, et al. Bismuth-213 for targeted radionuclide therapy: from atom to bedside. Pharmaceutics. 2021;13:1–25. doi: 10.3390/pharmaceutics13050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Huang C-Y, Clarke R. Targeted alpha anticancer therapies: update and future prospects. Biol Targets Ther. 2014;8:255. doi: 10.2147/BTT.S29947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47:934–946. doi: 10.1007/s00259-019-04567-2. [DOI] [PubMed] [Google Scholar]
- Beyer GJ, Bergmann R, Schomäcker K, Rösch F, Schäfer G, Kulikov EV, et al. Comparison of the biodistribution of 225 Ac and radio-lanthanides as citrate complexes. Isot Isot Environ Heal Stud. 1990;26:111–114. doi: 10.1080/10256019008624245. [DOI] [Google Scholar]
- Boden S, Vints K, Cagno S, Maertens D, Van Hecke K, Cardinaels T. Thorium-229 quantified in historical Thorium-228 capsules. Appl Radiat Isot. 2017;120:40–44. doi: 10.1016/J.APRADISO.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003;63:5084–5090. [PubMed] [Google Scholar]
- Cassells I, Ahenkorah S, Burgoyne AR, Van de Voorde M, Deroose CM, Cardinaels T, et al. Radiolabeling of human serum albumin with terbium-161 using mild conditions and evaluation of in vivo stability. Front Med. 2021;8:1–12. doi: 10.3389/fmed.2021.675122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo Seoane D, de Saint-Hubert M, Crabbe M, Struelens L, Koole M, et al. Targeted alpha therapy: a critical review of translational dosimetry research with emphasis on actinium-225. Q J Nucl Med Mol Imaging. 2020;64:265–277. doi: 10.23736/S1824-4785.20.03266-5. [DOI] [PubMed] [Google Scholar]
- Cordier D, Forrer F, Bruchertseifer F, Morgenstern A, Apostolidis C, Good S, et al. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8, Met(O2)11]- substance P: A pilot trial. Eur J Nucl Med Mol Imaging. 2010;37:1335–1344. doi: 10.1007/s00259-010-1385-5. [DOI] [PubMed] [Google Scholar]
- de Kruijff RM, Wolterbeek HT, Denkova AG. A critical review of alpha radionuclide therapy-how to deal with recoiling daughters? Pharmaceuticals. 2015;8:321–336. doi: 10.3390/ph8020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecoll E, Gaertner FC, Miederer M, Blechert B, Vallon M, Müller JM, et al. Treatment of peritoneal carcinomatosis by targeted delivery of the radio-labeled tumor homing peptide 213Bi-DTPA-[F3]2 into the nucleus of tumor cells. PLoS ONE. 2009;4:e5715. doi: 10.1371/journal.pone.0005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryilmaz K, Kilbas B. Detailed chemistry studies of 225Actinium labeled radiopharmaceuticals. Curr Radiopharm. 2021 doi: 10.2174/1874471014666210528123936. [DOI] [PubMed] [Google Scholar]
- Hooijman EL, Chalashkan Y, Ling SW, Kahyargil FF, Segbers M, Bruchertseifer F, et al. Development of [225Ac]Ac-PSMA-I&T for targeted alpha therapy according to GMP guidelines for treatment of mCRPC. Pharmaceutics. 2021 doi: 10.3390/pharmaceutics13050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JM, Amor-Coarasa A, Sweeney E, Wilson JJ, Causey PW, Babich JW. A suitable time point for quantifying the radiochemical purity of 225Ac-labeled radiopharmaceuticals. EJNMMI Radiopharm Chem. 2021 doi: 10.1186/s41181-021-00151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–728. doi: 10.1007/s00259-019-04612-0. [DOI] [PubMed] [Google Scholar]
- Kozempel J, Mokhodoeva O, Vlk M. Progress in targeted alpha-particle therapy. What we learned about recoils release from in vivo generators. Mol A J Synth Chem Nat Prod Chem. 2018 doi: 10.3390/MOLECULES23030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106–2119. doi: 10.1007/s00259-014-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil C, Bruchertseifer F, Giesel F, Apostolidis C, Haberkorn U, Morgenstern A. Ac-225-DOTATOC—dose finding for alpha particle emitter based radionuclide therapy of neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2015;42:1–924. doi: 10.1007/s00259-015-3198-z. [DOI] [PubMed] [Google Scholar]
- Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac-PSMA-617 for PSMA targeting alpha-radiation therapy of patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- Kruijff RMD, Raavé R, Kip A, Molkenboer-Kuenen J, Morgenstern A, Bruchertseifer F, et al. The in vivo fate of 225Ac daughter nuclides using polymersomes as a model carrier. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-48298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miederer M, McDevitt MR, Sgouros G, Kramer K, Cheung N-KV, Scheinberg DA. Pharmacokinetics, dosimetry, and toxicity of the targetable atomic generator, 225Ac-HuM195, in nonhuman primates. J Nucl Med. 2004;45:129–137. [PubMed] [Google Scholar]
- Miederer M, McDevitt MR, Borchardt P, Bergman I, Kramer K, Cheung NKV, et al. Treatment of neuroblastoma meningeal carcinomatosis with intrathecal application of α-emitting atomic nanogenerators targeting disialo-ganglioside GD2. Clin Cancer Res. 2004;10:6985–6992. doi: 10.1158/1078-0432.CCR-04-0859. [DOI] [PubMed] [Google Scholar]
- Miederer M, Henriksen G, Alke A, Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R, et al. Preclinical evaluation of the α-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res. 2008;14:3555–3561. doi: 10.1158/1078-0432.CCR-07-4647. [DOI] [PubMed] [Google Scholar]
- Nedrow JR, Josefsson A, Park S, Bäck T, Hobbs RF, Brayton C, et al. Pharmacokinetics, microscale distribution, and dosimetry of alpha-emitter-labeled anti-PD-L1 antibodies in an immune competent transgenic breast cancer model. EJNMMI Res. 2017;7:57. doi: 10.1186/s13550-017-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Andujar A, Pibida L. Performance of CdTe, HPGe and NaI(Tl) detectors for radioactivity measurements. Appl Radiat Isot. 2004;60:41–47. doi: 10.1016/J.APRADISO.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Pretze M, Kunkel F, Runge R, Freudenberg R, Braune A, Hartmann H, et al. Ac-EAZY! towards GMP-compliant module syntheses of 225 Ac-labeled peptides for clinical application. Pharmaceuticals (basel) 2021;14:652. doi: 10.3390/PH14070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poty S, Carter LM, Mandleywala K, Membreno R, Abdel-Atti D, Ragupathi A, et al. Leveraging bioorthogonal click chemistry to improve 225 AC-radioimmunotherapy of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2019;25:868–880. doi: 10.1158/1078-0432.CCR-18-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AKH, Ramogida CF, Schaffer P, Radchenko V. Development of 225 Ac radiopharmaceuticals: TRIUMF perspectives and experiences. Curr Radiopharm. 2018;11:156–172. doi: 10.2174/1874471011666180416161908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Jaggi JS, O’donoghue JA, Ruan S, McDevitt M, Larson SM, et al. Renal uptake of bismuth-213 and its contribution to kidney radiation dose following administration of actinium-225-labeled antibody. Phys Med Biol. 2011;56:721–733. doi: 10.1088/0031-9155/56/3/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40:37–61. [PubMed] [Google Scholar]
- Song H, Hobbs RF, Vajravelu R, Huso DL, Esaias C, Apostolidis C, et al. Radioimmunotherapy of breast cancer metastases with alpha-particle-emitter 225Ac: comparing efficacy with 213Bi, 90Y. Cancer Res. 2009;69:8941. doi: 10.1158/0008-5472.CAN-09-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Relative counting efficiency of the GC as function of sample volume for the quantification of 225Ac using the 213Bi and 221Fr EW. Design and results of an additional experiment to study the effect of sample volume on the relative GC detector efficiency.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.