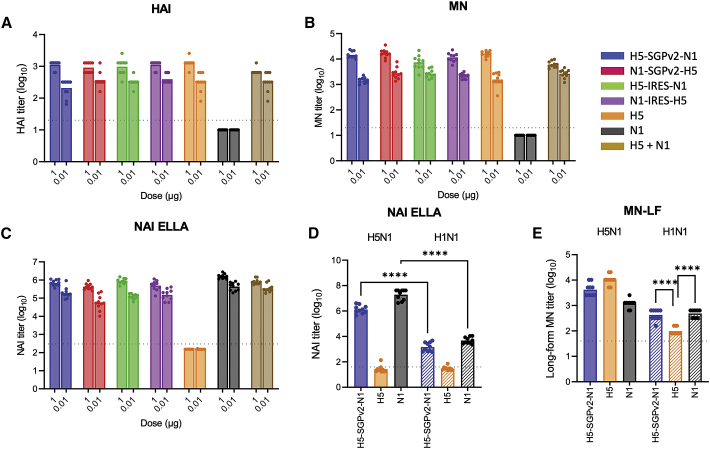
Figure 2.
sa-mRNA bicistronic A/H5N1 vaccines induce potent anti-HA and anti-neuraminidase neutralizing titers and cross-reactive response to heterologous A/H1N1 virus in BALB/c mice
Female BALB/c mice, 8–10 weeks old, were immunized (10 mice/group) on days 1 and 22 with bilateral 50 μL intramuscular injections in the rear quadriceps. Serum samples were obtained from bleed-outs of euthanized animals on day 43. Monocistronic and bicistronic sa-mRNA vaccines with different antigen orders were evaluated for their ability to induce anti-H5 neutralizing antibody responses by (A) hemagglutination inhibition (HAI) assay, (B) microneutralization (MN) assay, and (C) anti-N1 neutralizing antibody responses by neuraminidase inhibition (NAI) enzyme-linked lectin assay (ELLA). Cross-reactive anti-NA antibodies against heterologous strain A/H1N1 (A/Delaware/55/2019, striped bars) were examined by NAI ELLA (D) and microneutralization long form (MN-LF, E). Bars represent the geometric mean titer, with each dot denoting an individual titer. n = 10, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.