Abstract
Purpose
To define the impact of the COVID-19 outbreak on hospital surgical activity and assess the incidence of perioperative COVID-19 within two protocolized screening pathways for elective and non-elective surgery.
Methods
We conducted a prospective cohort study of adults undergoing surgery during the COVID-19 outbreak. The elective pathway included telephone surveys and a quantitative polymerase-chain-reaction test (RT-PCR) only for patients who were asymptomatic and at low risk of infection. Only patients with negative screening underwent surgery. In the non-elective pathway, preoperative screening was performed during the hospital admission.
Results
Among 835 patients considered for the elective pathway, 725 had negative RT-PCR results and underwent surgery. This reflects an 83% reduction in surgical activity from 2019. Moreover, 596 patients underwent non-elective surgery, representing a 28% reduction. Preoperatively, 39 patients (6.5%) tested positive for SARS-CoV-2 and underwent surgery through the non-elective pathway, vs. none in the elective pathway (p < 0.001). Postoperatively, 1.4% of elective surgery patients and 2.2% of non-elective surgery patients tested positive (p > 0.05). Mortality was higher in non-elective surgery (0.6% vs. 2.9%, p < 0.001) and in patients with COVID-19 (0% vs. 14%, p < 0.001).
Conclusions
The low incidence of COVID-19 in elective surgeries during the outbreak demonstrates the importance and effectiveness of preoperative screening, combining surveys and RT-PCR.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00595-022-02610-8.
Keywords: Perioperative, SARS-CoV-2, COVID-19, Nosocomial infection, Surgical
Introduction
Subsequent to the 2019 coronavirus disease (COVID-19) being declared a pandemic by the World Health Organization (WHO) on January 30, 2020 [1], many hospitals became exclusive COVID-19 centers, thereby limiting access to health care for patients who did not have COVID-19. As a result, elective surgical activity decreased drastically worldwide, in an attempt to allocate the finite resources to COVID-19 patients [2]. However, some surgeries cannot and should not be substantially delayed.
As the first COVID-19 wave started to decline, hospitals were no longer exclusively or predominantly committed to COVID-19 patients and began considering the needs of non-COVID patients’. This situation compelled anesthesiology and surgical departments to establish pathways to detect and prevent the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) nosocomial infection to keep healthcare providers safe. Despite limited evidence of the safety of anesthesia and surgery for COVID patients, early reports showed an increased risk of postoperative complications and mortality among patients with severe perioperative SARS-CoV-2 [3]. It remained unclear whether non-emergent surgery should be delayed in all patients with confirmed or suspected SARS-CoV-2 infection. The identification of asymptomatic and mild cases is challenging but important, since perioperative stress may exacerbate a current infection and result in fatal postoperative complications [4]. Although the incidence of asymptomatic COVID-19 ranged from 5% in the community [5] to 14% in women admitted for delivery [6], there were no such data in the general surgical population.
Strategies aimed at maintaining “COVID-free” surgical facilities have been reported, including the early detection of asymptomatic patients; however, the effectiveness of such strategies and their impact on patient outcomes remain unclear [7–9]. We conducted this study primarily to define the impact of the COVID-19 outbreak on surgical activity in a tertiary hospital caring for a population with a high prevalence of SARS-CoV-2. Second, we assessed the incidence of perioperative COVID-19 diagnosis and nosocomial infection within two COVID-19 protocolized screening pathways: for elective and non-elective surgery. Finally, we evaluated whether patients with a perioperative COVID-19 diagnosis were at increased risk of postoperative complications compared with patients who did not have COVID-19.
Methods
Study design and participants
We conducted a prospective single center cohort study of adult patients undergoing surgery at Hospital Clinic of Barcelona (Catalonia), during the first weeks of the COVID-19 outbreak in Barcelona, between March 16 and May 25, 2020. The study was approved by Hospital Clinic of Barcelona Institutional Review board (HCB/2020/0433) with individual consent waived.
The Hospital Clinic of Barcelona (HCB) is a public University Hospital with 713 beds, including 48 intensive care unit (ICU) beds, serving as a community hospital for a population of 540,000 people. On February 15, 2020, the first patient with COVID-19 was admitted to HCB. On March 16, HCB implemented a contingency plan which included the designation of a committee comprising surgeons, anesthesiologists, and nurse managers entitled to develop a prioritization strategy according to diagnoses and surgery urgency, and to perform a day-to-day case-schedule review considering the hospital COVID-19 load. During this period, only disorders that could not be deferred for more than 30 days were approved. As part of this strategy, a screening protocol for SARS-CoV-2 infection was implemented.
Protocol
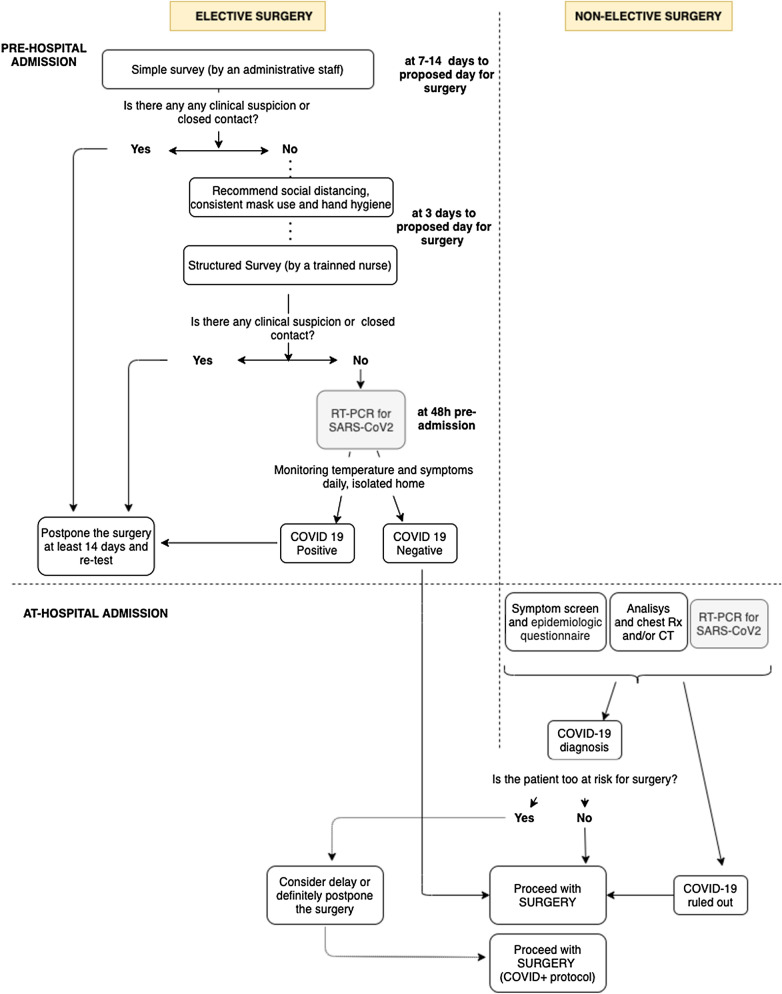
An elective pathway (Fig. 1) was designed for patients whose condition acuity allowed for the delay of surgery by at least 30 days without impacting the surgical outcome or disease process significantly, and who could be contacted before presenting for admission. First, a telephone survey was conducted by an administrative staff member 7–14 days before scheduled surgery, to postpone the procedure by at least 14 days if any clinical signs or symptoms of SARS-CoV-2 infection were suspected in the patient or their close contacts. The non-excluded patients were educated about the importance of social distancing, the consistent wearing of masks, and hand hygiene. Three days prior to surgery, a specific structured questionnaire (Supplemental Table 1) developed by the Spanish Association of Surgeons [10] was conveyed over the phone by a trained nurse, to identify patients at risk of having COVID-19 signs or symptoms. Elective surgery was postponed by at least for 14 days if patients or their immediate contacts had confirmed or suspected COVID-19. Patients who remained asymptomatic 48 h before scheduled surgery and who were evaluated as being at low risk of exposure were assessed for SARS-CoV-2 infection with a quantitative polymerase-chain-reaction test (RT-PCR) through a nasopharyngeal swab. To avoid further infection, we recommended strict isolation from when the nasopharyngeal swab was taken until hospital admission. Only patients whose screening was negative were scheduled for surgery.
Fig. 1.
Screening pathways for elective and non-elective surgery
The non-elective pathway (Fig. 1) was designed for patients whose indication for surgery was established only after hospital admission, if postponing surgery was medically unreasonable, or if surgery was urgent. COVID-19 screening was performed at the time of hospital admission. Patients were considered COVID-19 positive if clinical, radiological, or laboratory criteria were met (Supplemental Table 2). Patients with a negative COVID-19 test result had surgery through the “clean” path, including routine operating rooms, the post-anesthesia care unit (PACU), and surgical wards. Patients with a positive COVID test result underwent surgery on a COVID-19 path, including designated operating rooms, PACU, and surgical wards. Both paths were in the same building but in different pavilions. Moreover, health care providers worked exclusively in either one of the areas, reducing the risk of transmission. For patients with multiple surgeries during the same hospitalization, only the first operation was considered. Postoperative RT-PCR was not performed unless the patients developed signs or symptoms of COVID-19. Routine follow-up was conducted by phone 15 days after surgery.
Outcomes
Our primary outcome was to evaluate the impact of the COVID-19 outbreak on the surgical activity in a tertiary hospital caring for a population with a high prevalence of SARS-CoV-2. The secondary outcomes were the incidence of preoperative COVID-19 diagnosis according to the screening criteria (Supplemental Table 2) among patients scheduled for elective and non-elective surgery; and the incidence of postoperative COVID-19 diagnosis during the initial 15 postoperative days. The exploratory outcome was the incidence of postoperative complications and mortality among the COVID-19 positive patients.
Measurements
Qualifying patients were identified from the hospital surgical schedule database (SAP). Collected data included: demographic and morphometric information; comorbidities; surgical specialty; perioperative clinical, radiological, and laboratory findings compatible with SARS-CoV-2 infection; and postoperative complications and mortality within 15 postoperative days. We reviewed the patients’ electronic medical records in SAP. Each suspected COVID-19 case was assessed independently by two adjudicators (RP and ER). Non-consensus and all positive cases were adjudicated by the senior investigator (GMP).
Statistical analysis
Elective and non-elective pathways were compared by the T test or chi-square test, as appropriate. Data are expressed as means ± standard deviation and number (%), as appropriate. A Type 1 error was set at < 5%. For data presentation purposes only, patients are grouped according to the pandemic phases established by the Spanish government. These phases were defined according to the percentage of COVID-19-related hospital admissions among the total number of hospitalized patients: phase I, nearly normal scenario (less than 5% COVID-19-related admissions); phase II, low level alert (5–25%); phase III, medium level alert (25–50%); phase IV, high level alert (50–75%); and phase V, emergency scenario with more than 75% COVID-19-related admissions [11].
Results
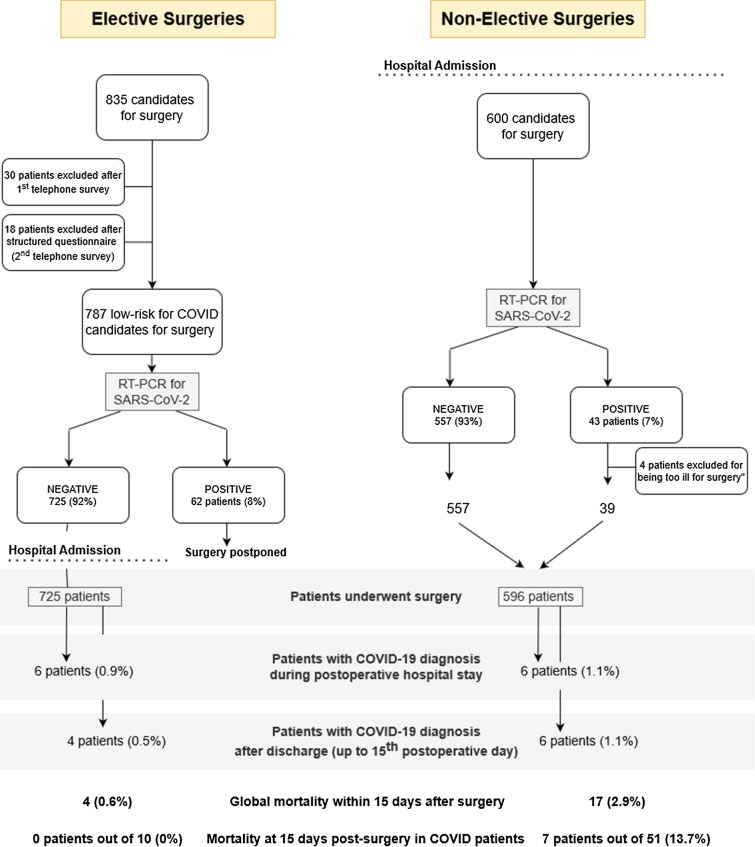
In the 10-week period from March 16th to May 25th 2020, 2404 patients with COVID-19 were admitted to HCB. COVID-19 dedicated wards were progressively opened and up to 130 ICU beds were allocated according to this overflow. At peak workload, 85% of patients admitted to the hospital were diagnosed with a COVID-19-related disease. During this period, 835 patients were considered for surgery through the elective pathway (Fig. 2). Among these patients, 30 were excluded, because they were at high risk of having COVID-19 or SAR-CoV-2 infection according to the results of the first telephone survey. An additional 18 patients were excluded later by the second structured questionnaire performed 3 days before surgery. Therefore, those 48 surgeries were postponed. Of the remaining 787 asymptomatic patients at low risk for contact (92%), 725 had a negative RT-PCR test result and underwent surgery during this period. Compared with the 4389 elective surgeries performed during the same period in 2019, this represents an 83% reduction in elective surgeries during the COVID-19 pandemic outbreak.
Fig. 2.
Flowchart of patient cadidates for elective and non-elective surgery
Six hundred patients were admitted to the hospital to undergo scheduled surgery following the non-elective pathway (Fig. 2). Of these 600 patients, 557 (93%) were negative for SARS-CoV-2. Of the 43 positive patients, 4 multimorbid orthopedic patients with COVID-19 diagnosis had their surgery postponed, because they were considered too frail. The remaining 596 patients underwent surgery on the non-elective pathway. This represents a 28% (596/833) reduction in the number of non-elective surgeries compared with the similar period in 2019. Table 1 summarizes the demographic, clinical, and surgical characteristics of the 1321 patients who underwent surgery and were included in both pathways.
Table 1.
Clinical and demographic characteristics by surgical pathway (n = 1321)
| Elective (n = 725) | Non-elective (n = 596) | P value | |
|---|---|---|---|
| Age, years | 61 ± 1 | 53 ± 1 | < 0.0001 |
| Sex, Male/Female | 344 (47)/381 (53) | 204 (34)/392 (66) | < 0.0001 |
| ASA, 1–2/3–4 | 479 (66)/246 (34) | 411 (69)/185 (31) | ns |
| HTA | 298 (41) | 184 (31) | < 0.001 |
| BMI, Kg/m2 | 28 ± 5 | 27 ± 6 | ns |
| Surgical Specialties | < 0.0001 | ||
| General Surgery | 157 (22) | 95 (16) | |
| Urology-Gynecology | 209 (29) | 25 (4) | |
| Cardio-thoracic | 104 (14) | 22 (3) | |
| Vascular | 21 (3) | 12 (2) | |
| Obstetrics | 21 (3) | 225 (38) | |
| Neurosurgery | 42 (6) | 34 (6) | |
| Orthopedics -Traumatology | 27 (4) | 138 (23) | |
| Otorhinolaryngology | 39 (5) | 10 (2) | |
| Others | 105 (14) | 35 (6) | |
| Preoperative COVID-19 positive diagnosis | 0 (0) | 39 (6.5) | < 0.0001 |
| Postoperative COVID-19 positive diagnosis | 6 (0.9)b | 6 (1.1)c | ns |
| Post-discharge COVID-19 positive diagnosis | 4 (0.5)d | 6 (1.1)e | ns |
| Mortality | 4 (0.6) | 17 (2.9) | < 0.0001 |
| COVID-19 Mortality | 0 | 7 |
Data are expressed as means ± SD, or n (%)
BMI body mass index, ASA American Society of Anesthesiology physical status. Others miscellaneous major surgeries
b 6 patients among the 717 assessed
c 6 patients with COVID-19 diagnosis among the 596 assessed
d 4 patients with COVID-19 diagnosis among the 692 assessed
e 6 patients with COVID-19 diagnosis among the 538 assessed
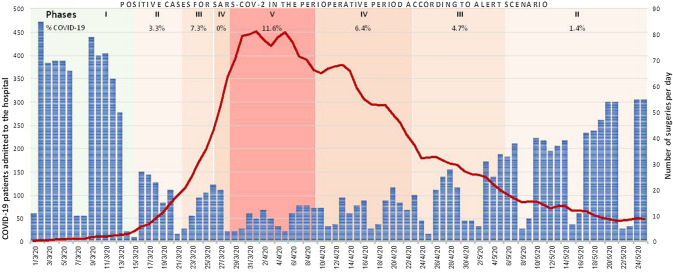
During this time interval and according to the dynamic scenarios proposed by the Spanish Society of Surgeons [11], the number of surgeries performed was inversely proportional to the percentage of patients hospitalized with COVID-19-related disease (Fig. 3). We observed an exponential decrease in the number of surgical procedures moving from phase II (low level alert scenario) to phase V (emergency scenario) achieving a 35% difference between those phases. Similarly, we observed an inverse increase in surgical activity moving back from phase V to phase II (Fig. 3). Concomitantly, there was a progressive increase in perioperative COVID-19 cases, from a 3.3% incidence in phase II to an 11.6% in phase V (Fig. 3). Thirty-nine patients (6.5%) underwent surgery in the non-elective pathway despite a diagnosis of COVID-19, compared with none in the elective pathway, p < 0.001. Notwithstanding, 10 (1.4%) patients in the elective pathway and 12 (2.2%) in the non-elective pathway became COVID-19 positive within the first 15 postoperative days (non-significant difference). Among those, 4 (0.5%) patients who had surgery in the elective and 6 (1.1%) in the non-elective pathway were diagnosed after hospital discharge (Fig. 2). The median time from surgery to COVID-19 diagnosis was 3 (range 1–7) days.
Fig. 3.
Number of surgeries and COVID-19 patients admitted to the hospital and diagnosed during the perioperative period according to alert scenario. % COVID-19, percentage of cases diagnosed in the perioperative period (from admission to 15 day post-surgery) per total of surgical procedures performed in a concrete alert scenario previously defined. Phase I, almost normal scenario <5% COVID-19-related admissions to ward and ICU; Phase II, low level alert scenario 5–25% COVID-19-related admissions; Phase III, medium level alert scenario 5–25% COVID-19-related admissions; Phase IV, high level alert scenario 50–75% COVID-19-related admissions; Phase V, emergency scenario >75% COVID-19-related admissions. Blue columns are total of surgeries (elective and non-elective) performed per day. Red line is the total COVID-19 patients admitted to the hospital
The mortality rate was higher in the non-elective surgery group (0.6% vs. 2.9%, p < 0.001) (Fig. 2 and Table 2). Seven of the 17 (41%) non-elective patients who died had a COVID-19 diagnosis. There were no deaths attributable to SARS-CoV-2 infection among the patients who underwent elective surgeries. Overall, patients with a perioperative COVID-19 diagnosis were older and had more comorbidities (Supplemental Table 3), and significantly more postoperative complications (40/61 patients, 66%) than the non-COVID-19 patients (227/1260 patients, 18%) including higher mortality (11% vs. 1%) (p < 0.0001, each) (Table 2). Globally, 67% (26/39) of patients with preoperative diagnosis of COVID-19 suffered a postoperative complication. This result is not significantly different from the complication rate of 64% (14/22) observed in patients diagnosed with COVID-19 postoperatively. Among the non-elective patients only, 26 of the 33 COVID-19 positive patients who had a postoperative complication were diagnosed with COVID-19 before surgery, which was not different from the complication rate of 58% (7/12) among patients with a postoperative COVID-19 diagnosis.
Table 2.
Incidence of postoperative complications in patients with vs. those without a perioperative COVID-19 diagnosis
| A. Total surgical population (n = 1321) | |||
|---|---|---|---|
| COVID-19 patients (n = 61) | Non COVID-19 patients (n = 1260) | P value | |
| Patients with postoperative complications, n (%) | 40 (66) | 227 (18) | < .001 |
| COVID-Pneumonia | 17 (28) | 0 | |
| Respiratory failure | 14 (23) | 36 (3) | |
| Cardiovascular | 16 (26) | 54 (4) | |
| Renal failure | 13 (21) | 45 (4) | |
| DVT/PE | 6 (10) | 11 (1) | |
| Neurological | 8 (13) | 34 (3) | |
| Surgical infection | 8 (13) | 41 (3) | |
| Urinary infection | 5 (8) | 35 (3) | |
| Septic shock | 11 (18) | 36 (3) | |
| Re-intervention | 4 (7) | 35 (3) | |
| Mortality | 7 (11) | 14 (1) | < .001 |
| B. Patients who underwent non-elective surgery (n = 596) | |||
|---|---|---|---|
| COVID-19 patients (n = 51) | Non COVID-19 patients (n = 545) | P value | |
| Patient with postoperative complications, n (%) | 33 (65) | 121 (22) | < .001 |
| COVID-Pneumonia | 12 (24) | 0 | |
| Respiratory failure | 15 (29) | 18 (3) | |
| Cardiovascular | 13 (25) | 29 (5) | |
| Renal failure | 12 (24) | 25 (5) | |
| DVT/PE | 3(6) | 7 (1) | |
| Neurological | 7 (14) | 24 (4) | |
| Surgical infection | 7 (14) | 22 (4) | |
| Urinary infection | 5 (10) | 26 (5) | |
| Septic shock | 12 (24) | 26 (5) | |
| Re-intervention | 3 (6) | 23 (4) | |
| Mortality | 7 (14) | 10 (2) | < .001 |
Data are presented as n (%)
DVT/PE deep venous thrombosis/pulmonary thromboembolism
Discussion
The growing requirement for COVID-19 dedicated wards and ICU beds during the COVID-19 outbreak reduced surgical activity worldwide [2, 12]. In HCB, during the 10 weeks of the first wave of the COVID-19 outbreak, the percentage of COVID-19 dedicated hospital beds ranged from 5% to 85% both in surgical wards and the ICU. Positivity for SARS-CoV-2 was confirmed in 56,000 people during this time in Catalonia alone. This incidence was accompanied by a marked reduction in surgical activity, especially in the emergency scenario (phase V).This marked decrease in surgical activity was also described by the COVID collaborative group, who predicted that the global 12-week cancellation would be 82% for benign disease surgery and 38% for cancer surgery [2]. Our data suggest that these figures might be even worse with an 83% reduction in elective surgery including cancer surgery. About 3 weeks after that peak, we attempted to resume surgical activity progressively, although this process took up to 20 weeks. Moreover, surgery cancellation did not end with the first wave; the surgery rate decreased again with the recurrent waves, although only elective surgery for benign diseases was affected. Although the cumulative impact of surgery cancellations is still to be revealed, it will certainly add to the already busy waiting lists. It is worth noting that the consequences are not merely quantitative, since the surgery cancellations and longer waiting list times will negatively affect the prognosis of many patients. To avoid unnecessary delays that might affect prognosis [12], while preventing COVID-19 nosocomial infections and protecting health care providers, we designed two dedicated perioperative pathways. The unique characteristics of SARS-CoV-2 infection, including high infectibility, long incubation period, and nonspecific symptoms at disease onset [13] contribute to the difficulty in detection of asymptomatic patients and those within the incubation period.
Contrary to dedicated ICUs, the PACU and operating rooms are semi-open spaces with complex air-filtration systems, so that containment of potential spread is nearly impossible, which presents further challenges. Previous reports on perioperative COVID pathways in orthopedic surgery [14], neurosurgery [9], and general surgery [15], also followed existing guidelines [15–17] and excluded COVID-19 elective cases to minimize the possibility of nosocomial transmission, and to reduce postoperative complications [15, 16]. Moreover, all guidelines advocate for a strict preoperative COVID-19 questionnaire [6, 9, 14, 18, 19] and RT-PCR screening [20] as the key to pathway success. However, they provide only limited evidence of the pathway effectiveness in preventing nosocomial and health care providers’ infection [6, 9, 14].
The current cohort of 1321 patients is one of the largest databases evaluating perioperative SAR-CoV-2 infection during a peak period of the pandemic in a population with high COVID-19 prevalence. We demonstrated that structured preoperative screening utilizing phone questionnaires can save as many as 5% of RT-PCR tests by detecting patients at risk of having COVID-19. Considering the relatively short time from surgery to the postoperative diagnosis of COVID-19 (median 3 days), it is reasonable to assume that some of these patients were already asymptomatic carriers or within the silent incubation period and missed by the RT-PCR screening, rather than that the contracted nosocomial infection. These results contradict those of Schlosser et al.[7] who found no differences between universal testing and testing according to exposure risk, in two cohorts of about 60,000 patients scheduled for elective surgery. However, in that report, the rate of preoperative RT-PCR was 48% in the universal testing strategy group and 23% in the per-risk strategy group, vs. 100% in our elective surgery population. Moreover, Schlosser et al. did not report on postoperative follow-up, so asymptomatic patients who become positive only after surgery, because of the long incubation period, cannot be assessed. Finally, the report by Schlosser et al.[7] included a population with low SARS-CoV-2 prevalence, whereas our study included scheduled surgeries within a period of peak incidence of COVID-19 cases in Barcelona, which was considered severely affected by the COVID-19 pandemic.
On the non-elective pathway, the incidence of preoperative COVID-19 infection was 7%, which is similar to the incidence reported in the Barcelona population during those weeks [21]. The similar incidence of postoperative COVID-19 diagnosis in the elective and non-elective groups further emphasizes the effectiveness of this screening protocol in detecting COVID-19 patients and in preventing nosocomial infection using designated operating rooms and PACU for COVID-19 patients.
After 2 years of the pandemic, numerous waves, and new SARS-CoV-2 variants, the situation has changed. The use of rapid antigen tests even considering their reduced sensitivity compared with RT-PCR [22] play an important role as a first-line or mass community screening test, especially in patients at lower risk of COVID-related perioperative complications, such as those undergoing outpatient procedures, those at low surgical risk, and vaccinated patients. However, more evidence is needed for decision makers. It is notable that 16% of the anesthesiologists working in the COVID-designated areas were infected during these weeks. As expected, this rate is higher than the overall 10% infection rate among health care providers reported by the Spanish government, presumably since our anesthesiologists were more exposed to high-risk aerosol-generating procedures than were health care providers working in wards or emergency departments. To prevent further infections, we prioritized training for the correct use of personal protective equipment with the appropriate space for dressing and undressing, implemented weekly COVID19 screening for professionals in the front-line, and limited the number of people allowed in common areas without masks, for example, at meal break times.
Finally, previous reports described nearly double the incidence of postoperative complications and higher mortality of COVID-19 patients [3, 12, 23]. However, our sample size was small and our populations were not comparable (Supplemental Table 3). Interestingly, the rate of complications was similar in patients with COVID-19 diagnosed preoperatively and those diagnosed postoperatively, again highlighting the importance of preoperative screening for minimizing viral spread and reducing the risk of postoperative complications.
Limitations
The main limitation of the current report was its single-center origin, in a tertiary hospital during the pandemic peak, making the results difficult to generalize for populations with lower COVID-19 prevalence. Furthermore, the 2% incidence of postoperative new SARS-CoV-2 infections might be underreported given that only patients presenting with clinical suspicion were tested postoperatively. Thus, asymptomatic patients might have not been detected. Finally, we were underpowered to detect an association between postoperative complications and SARS-CoV-2 infection.
Despite these limitations, this report provides valuable information about the impact of the COVID-19 pandemic peak on surgical activity. The low incidence of postoperative COVID-19 diagnosis in elective surgeries (1.4%) during the first weeks of the outbreak reinforces the use of these pathways and demonstrates the efficacy of systematic preoperative screening for SARS-CoV-2 infection, including not only RT-PCR, but also structured questionnaire-based screening.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Hospital Clínic Perioperative COVID Group: Aureli Torner; Francisco Carmona; Elena Sandoval; Eduard Quintana; Anna Carreras; Alfonso Alías; Juan López; Raul Martos; Francesc-Xavier Avilés; Mauricio López-Chacón; Gaspar Mestres; Vicenç Riambau; Marina Cámara; Ramón Sieira; Carlos Guerrero; Leandro Grando; Alberto Di Somma; Luís Reyes; Ramón Llul; Constantino Fondevila; Joan Beltran; Josep Gracia; Montserrat Tio; Annabel Blasi; Jaume Balust; Ricard Valero; Enrique Carrero; Jaume Fontanals; Montserrat Celemin; M Teresa Anglada; Xavier Sala-Blanch; Oscar Comino; Bartolomeu Ramis; Ana Fernandez; Jorge Aliaga.
Funding
The study was not funded.
Declarations
Conflict of interest
We have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roger Pujol and Eva Rivas authors contributed equally to this manuscript.
References
- 1.World Health Organization G. World Health Organization (2020) Coronavirus disease 2019 (COVID-19) situation report–100.
- 2.CovidSurg Collaborative. Nepogodiev D, Bhangu A. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CovidSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aminian A, Safari S, Razeghian-Jahromi A, Ghorbani M, Delaney CP. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020;272:e27–e29. doi: 10.1097/SLA.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg B, Haddix M, Lee R, Butler-Wu S, Holtom P, Yee H, et al. Community prevalence of SARS-CoV-2 among patients with influenzalike illnesses presenting to a Los Angeles medical center in march 2020. JAMA NLM. 2020;323:1966–1967. doi: 10.1001/jama.2020.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlosser M, Signorelli H, Gregg W, Korwek K, Sands K. COVID-19 testing processes and patient protections for resumption of elective surgery. Am J Surg. 2020;221:49–52. doi: 10.1016/j.amjsurg.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hojaij FC, Chinelatto LA, Boog GHP, Kasmirski JA, Lopes JVZ, Sacramento FM. Surgical practice in the current COVID-19 pandemic: a rapid systematic review. Clinics. 2020;75:e1923. doi: 10.6061/clinics/2020/e1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spina A, Boari N, Gagliardi F, Bailo M, Calvanese F, Mortini P. The Management Of Neurosurgical Patients During The Covid-19 Pandemic. World Neurosurg. 2020;5–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875020308706 [DOI] [PMC free article] [PubMed]
- 10.Balibrea JM, Badia JM, Rubio Pérez I, Martín Antona E, Álvarez Peña E, García Botella S, et al. Surgical management of patients with COVID-19 infection. Recommendations of the Spanish association of surgeons. Cir Esp. 2020;98:251–259. doi: 10.1016/j.ciresp.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales-Conde S, Balla A, Álvarez Gallego M, Aranda Narváez JM, Badia JM, Balibrea JM, et al. A dynamic scale for surgical activity (DYSSA) stratification during the COVID-19 pandemic. Br J Surg. 2020;107:e425–e426. doi: 10.1002/bjs.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Søreide K, Hallet J, Matthews JB, Schnitzbauer AA, Line PD, Lai PBS, et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg. 2020 doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Y, Leng K, Shan L, Guo M, Zhou J, Tian Q, et al. A clinical pathway for pre-operative screening of COVID-19 and its influence on clinical outcome in patients with traumatic fractures. Int Orthop. 2020;44:1549–1555. doi: 10.1007/s00264-020-04645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936–e934. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffernan DS, Evans HL, Huston JM, Claridge JA, Blake DP, May AK, et al. Surgical infection society guidance for operative and peri-operative care of adult patients infected by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Surg Infect (Larchmt) 2020;21:301–308. doi: 10.1089/sur.2020.101. [DOI] [PubMed] [Google Scholar]
- 17.Germanò A, Raffa G, Angileri FF, Cardali SM, Tomasello F. COVID-19 and neurosurgery. Literature and neurosurgical societies recommendations update. World Neurosurg. 2020;139:e812–e817. doi: 10.1016/j.wneu.2020.04.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgi PD, Villa F, Gallazzi E, Debernardi A, Schirò GR, Crisà FM, et al. The management of emergency spinal surgery during the COVID-19 pandemic in Italy. Bone Joint J. 2020;102-B:671–676. doi: 10.1302/0301-620X.102B6.BJJ-2020-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolone S, Gambardella C, Brusciano L, del Genio G, Lucido FS, Docimo L. Telephonic triage before surgical ward admission and telemedicine during COVID-19 outbreak in Italy. Effective and easy procedures to reduce in-hospital positivity. Int J Surg. 2020;78:123–125. doi: 10.1016/j.ijsu.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Muharraqi MA. Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery - continuing the service and ‘suppressing’ the pandemic. Br J Oral Maxillofac Surg. 2020;58:503–505. doi: 10.1016/j.bjoms.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronda EEP. ESTUDIO ENE-COVID19: PRIMERA RONDA ESTUDIO NACIONAL DE SERO-EPIDEMIOLOGÍA DE LA INFECCIÓN POR SARS-COV-2 EN ESPAÑA INFORME PRELIMINAR 13 DE MAYO DE 2020. 2020.
- 22.Kim J, Sung H, Lee H, Kim J-S, Shin S, Jeong S, et al. Clinical performance of rapid and point-of-care antigen tests for SARS-CoV-2 variants of concern: a living systematic review and meta-analysis. Viruses. 2022;14:1479. doi: 10.3390/v14071479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Martino M, García Septiem J, Maqueda González R, Muñoz de Nova JL, de la Hoz RÁ, Correa Bonito A, et al. Elective surgery during the SARS-CoV-2 pandemic (COVID-19): a morbimortality analysis and recommendations on patient prioritisation and security measures. Cir Esp. 2020;98:525–532. doi: 10.1016/j.ciresp.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.