Extended Data Fig. 8. Ceramide supplementation eliminates the beneficial effects derived from intestinal AMPKα1 deficiency.
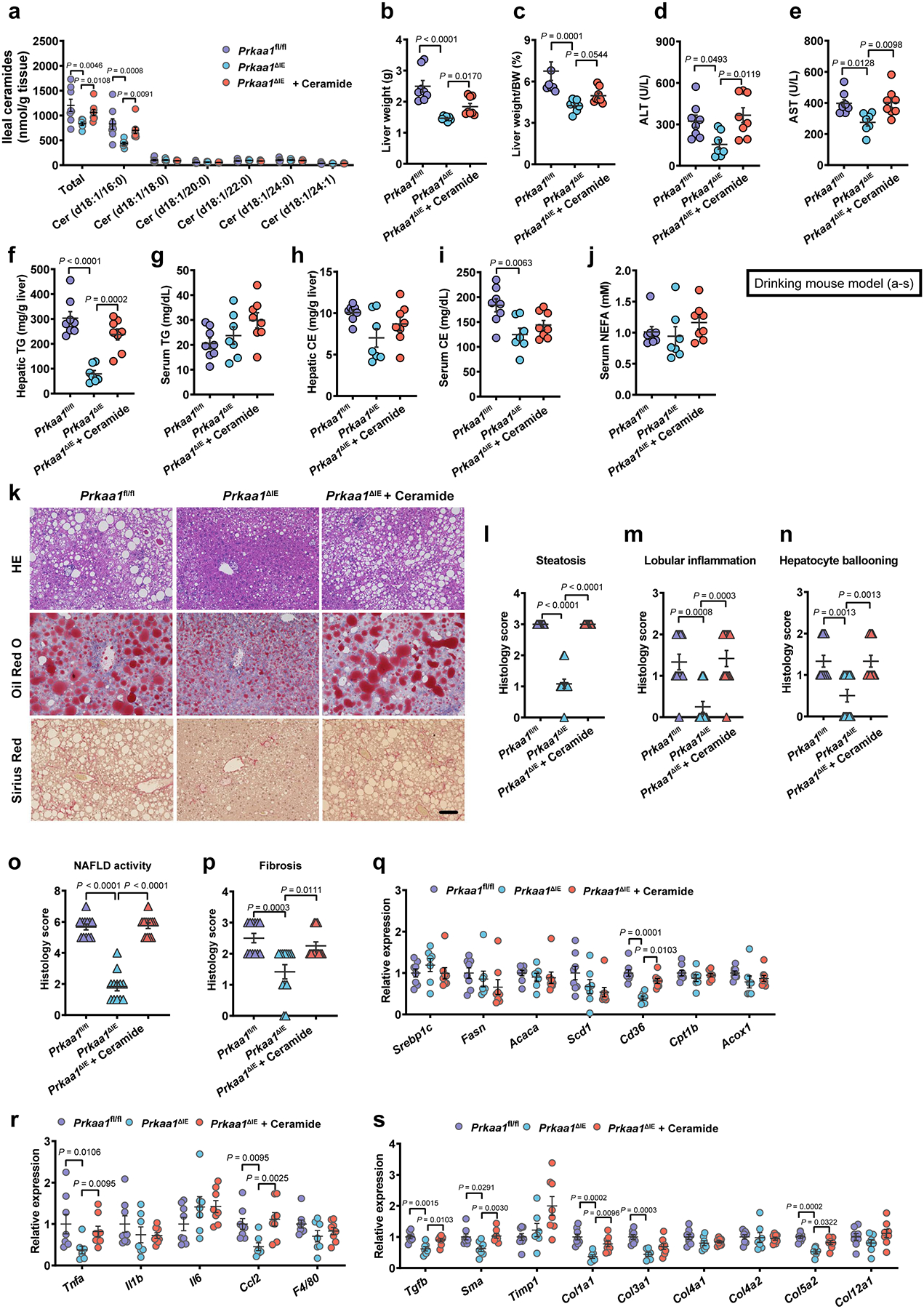
Eight-week-old male Prkaa1fl/fl and Prkaa1ΔIE mice were treated with or without 10 mg/kg ceramide (d18:1/16:0) by daily i.p. injection under HFHCD plus nicotine water treatment for 20 weeks (SPF, Prkaa1fl/fl, n = 8 mice; Prkaa1ΔIE, n = 7 mice; Prkaa1ΔIE + Ceramide, n = 8 mice). a, Ileal ceramide profiles. b, Liver weights. c, Liver weight–to–body weight ratios. d, e, Serum ALT (d) and AST (e) levels. f-j, Hepatic TG (f), serum TG (g), hepatic CE (h), serum CE (i) and serum NEFA (j) contents. k, Representative H&E staining (upper), Oil Red O staining (middle) and Sirius Red staining (lower) of liver sections (n = 4 mice/group, 3 images/mouse). Scale bar, 100 μm. l-p, Histology scores of steatosis (l), lobular inflammation (m), hepatocyte ballooning (n), NAFLD activity (o) and fibrosis stage (p). q-s, Relative mRNA levels of genes related to hepatic lipid metabolism (q), inflammation (r) and fibrosis (s). Data are the means ± s.e.m. d-g, i, One-way ANOVA with Tukey’s post hoc test. h, One-way ANOVA with Dunnett’s T3 post hoc test. a-c, j, l-s, Kruskal-Wallis test with Dunn’s test.
