Figure 1.
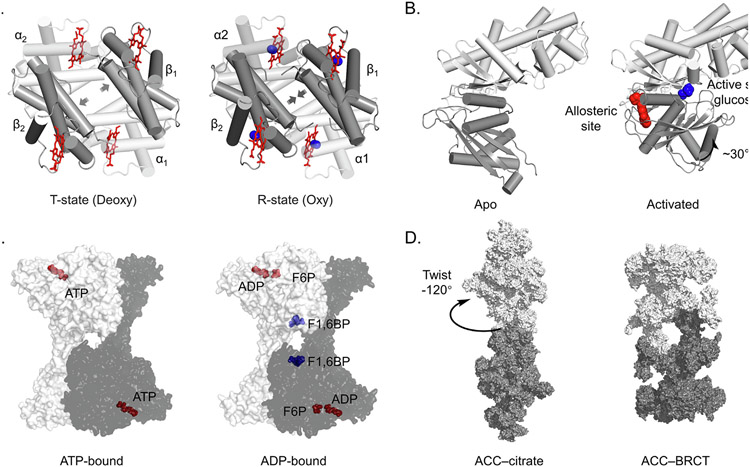
Allosteric regulation is common in biology and can be targeted with drugs. (A) Hemoglobin in the T (Tense, left) and R (Relaxed, right) conformations. The T conformation is stabilized in the absence of oxygen and has low binding affinity. Binding of oxygen transitions the tetramer to the R state, increasing oxygen binding affinity. PDB: 2HHB, 1HHO. α subunit in white, β in grey, heme in red, oxygen in blue. (B) GK changes conformation to become active when the allosteric site is bound. Glucose in blue, allosteric ligand in red. PDB: 1V4T, 1V4S. (C) Binding of F2,6BP to the allosteric site of the homotetrameric phosphofructokinase-1 (PFK1) complex causes rotation of the protomers relative to each other to stimulate enzymatic activity. ATP, ADP and F6P in red, F1,6BP in blue. PDB: 4XYJ, 4XZ2. (D) Citrate allosterically activates human acetyl-CoA carboxylase (ACC) by stabilizing filament formation. PDB: 6G2D,6G2I.