Extended Data Fig. 7 |. Additional characteristics of t-mOVA and demonstration of sialic acid at trophoblast membranes in contact with maternal blood.
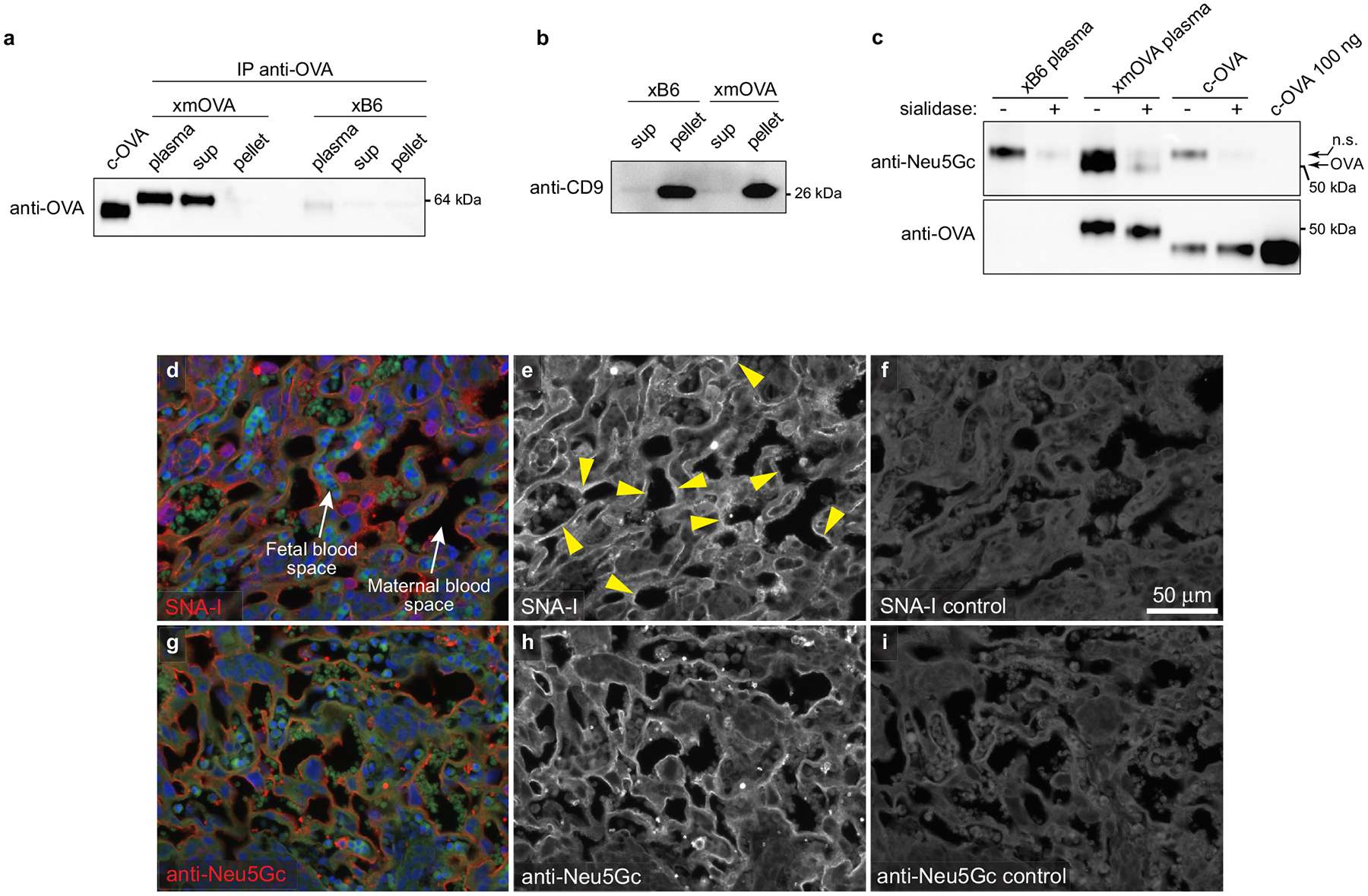
a, b, Shed t-mOVA is present within the non-pelletable, non-exosomal fraction of maternal plasma. Equal volumes of plasma from xB6- and xmOVA-mated mice (E18.5) were subjected to differential centrifugation (see Methods) followed by either anti-OVA immunoprecipitation and anti-OVA immunoblotting (a) or immunoblotting for the exosome-specific marker CD9 (b). We analyzed three different fractions: “clarified plasma” (i.e., the plasma after an initial low-speed (10,000×g) centrifugation), and the “sup” and “pellet” fractions from 110,000×g ultracentrifugation. Anti-OVA immunoblotting identified mOVA in both the clarified plasma and 110,000xg “sup” fractions, but not in the 110,000×g “pellet” fraction where exosomes reside, as demonstrated by the anti-CD9 immunoblotting. Data are representative of 3 (a) and 2 (b) separate experiments. c, Shed t-mOVA contains N-glycolylneuraminic acid (Neu5Gc), the sialic acid variant required for strong α(2,6)-Sia binding to mouse CD2252. Equal volumes of plasma respectively pooled from 3 xB6-mated and xmOVA-mated mice (E16.5–18.5) were subjected to differential centrifugation, anti-OVA immunoprecipitation on the 110,000×g supernatant, sialidase or mock digestion as indicated, and then anti-Neu5Gc immunoblotting. c-OVA was similarly immunoprecipitated and sialidase/mock-treated, or loaded directly onto the gel. As expected, Neu5Gc is absent from c-OVA. The non-specific (n.s.) band is likely free IgG heavy chain. Data are representative of 2 separate experiments. For gel source data, see Supplementary Fig. 1. d–i, Distribution of α(2,6)-Sia and Neu5Gc in the mouse placental labyrinth. Placental sections prepared from mice on E12.5 were stained with SNA-I (d–f) or anti-Neu5Gc antibodies (g–i). For the SNA-I control (f), the adjacent section was pretreated with neuraminidase A; for the anti-Neu5Gc control (i), free Neu5Gc was added in with the primary antibody. Fetal and maternal blood spaces were respectively identified by the presence of DAPI+ (blue counterstain) nucleated or DAPIneg enucleated RBCs, both of which are autofluorescent on the green channel. Note the SNA-I staining on trophoblast membranes in direct contact with maternal blood (arrowheads). This staining was not as continuous as the staining for Neu5Gc, which was present in all trophoblast membranes in contact with maternal blood. The small round structures showing strong Neu5Gc staining are morphologically consistent with platelets. Images are representative of 3 independent experiments.
