Table 2.
Summary of major marketed and clinically reported small molecule immunotherapy drugs (Up to March 2022).
| Target | Name | Structure | Company | Highest Development Phases |
|---|---|---|---|---|
| PD-L1/VISTA | CA-170 |
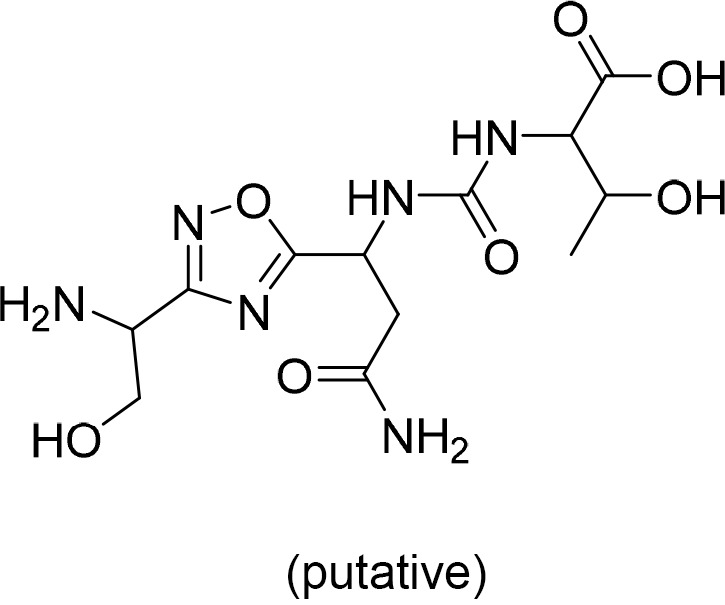
|
Aurigene, Curis | Phase II (NCT01288911) |
| PD-L1 | INCB-086550 |
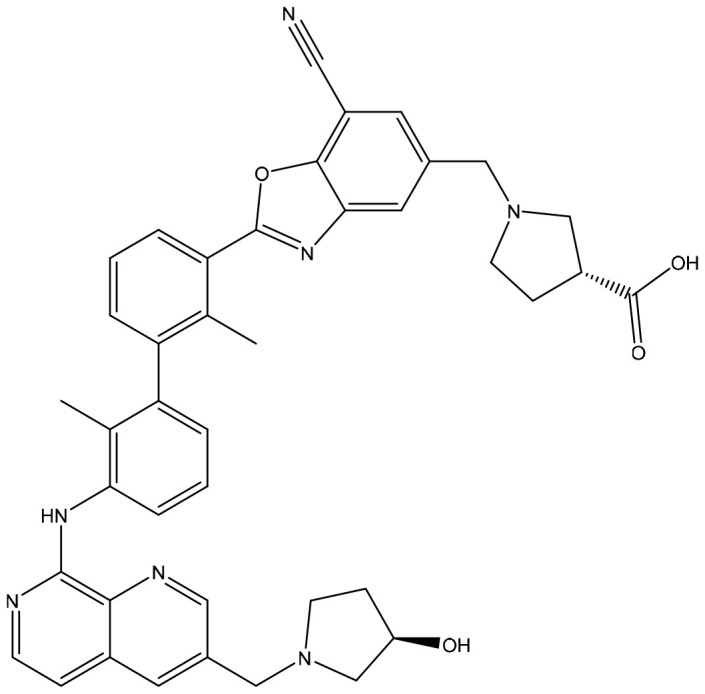
|
Incyte | Phase II (NCT04629339) |
| PD-L1 | GS-4224 |
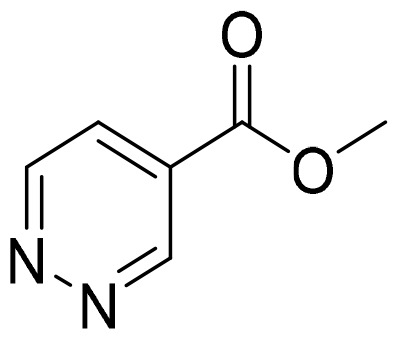
|
Gliead | Phase 1b/2 (NCT04049617) |
| PD-1 | MX-10181 | undisclosed | Maxinovel | Phase I (NCT04122339) |
| IDO1 | BMS-986205 |
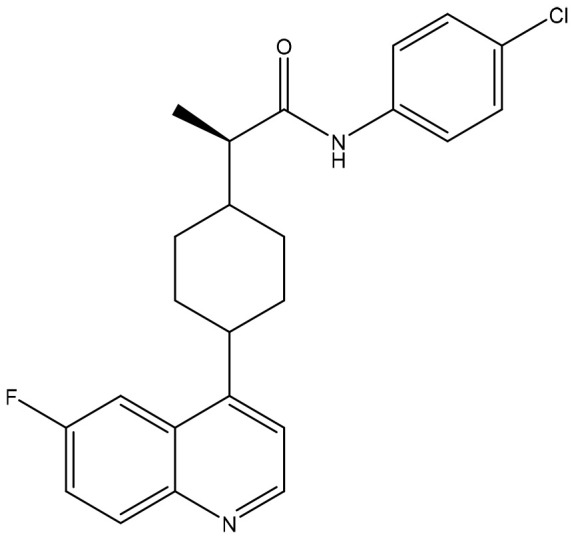
|
Bristol-Myers Squibb | Phase III (NCT03661320) |
| IDO1 | INCB-024360 |
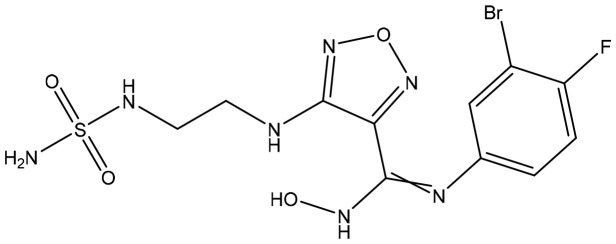
|
Incyte | Phase III (NCT02752074) |
| STING | ADU-S100 |
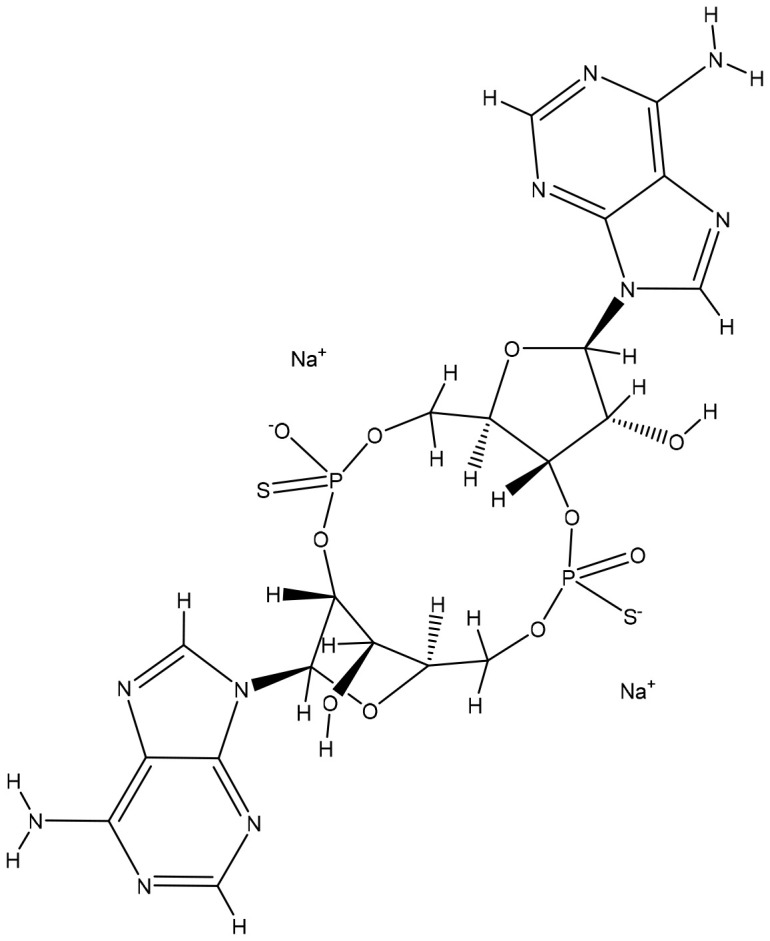
|
Aduro, Novartis | Phase II (NCT03937141) |
| STING | MK-1454 |
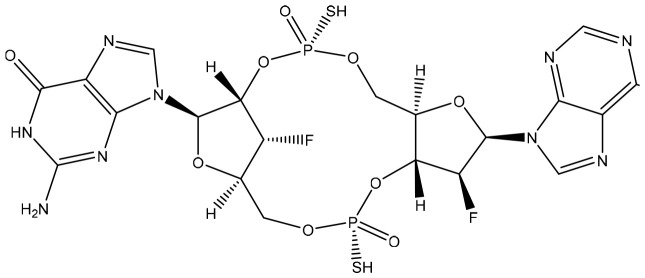
|
Merck | Phase II (NCT04220866) |
| A2AR | AZD4635 |
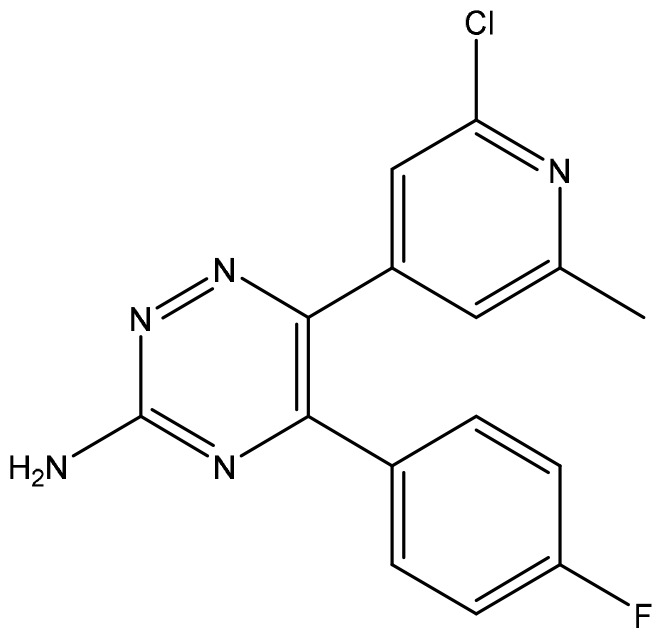
|
AstraZeneca | Phase II (NCT04089553) |
| A2AR | NIR178 |
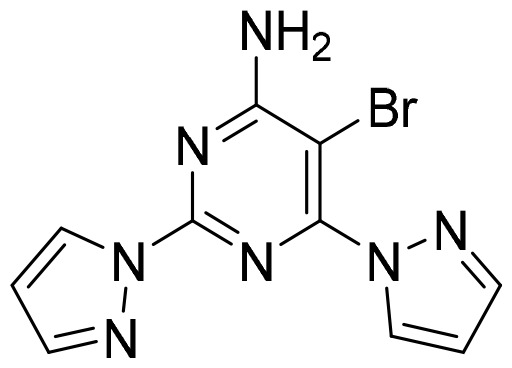
|
Novartis | Phase II (NCT03207867) |
| CXCR2 | AZD5069 |
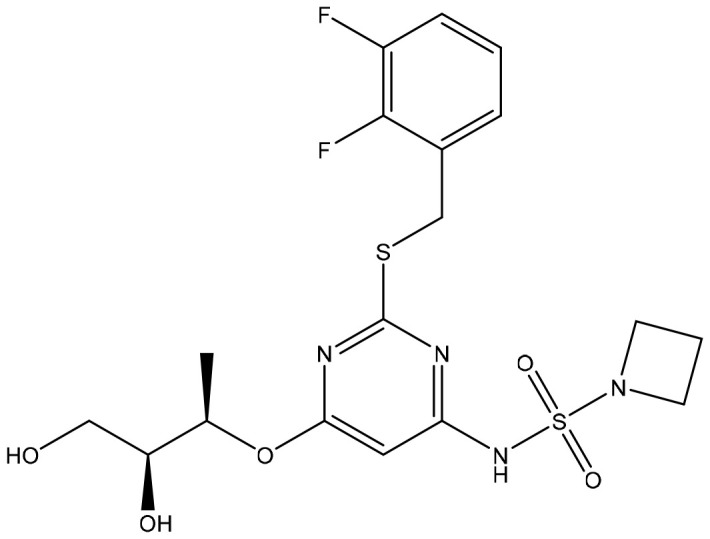
|
AstraZeneca | Phase II (NCT03177187) |
| CXCR4 | Mavorixafor |
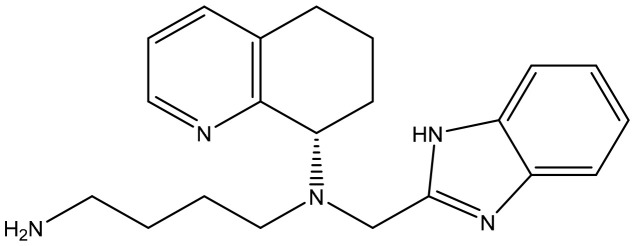
|
X4 Pharmaceuticals | Phase III (NCT03995108) |
| CCR2/5 | BMS-813160 |
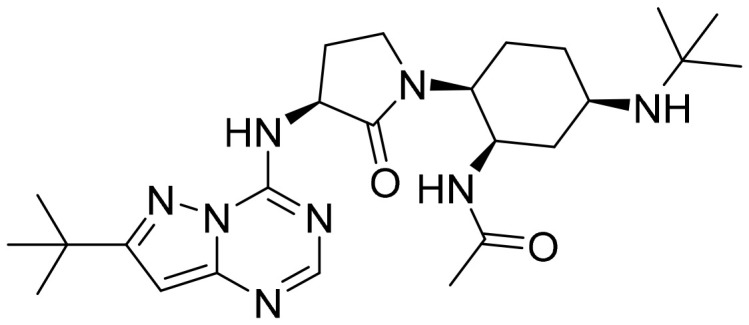
|
Bristol-Myers Squibb | Phase II (NCT03184870) |
| TLR7 | Imiquimod |
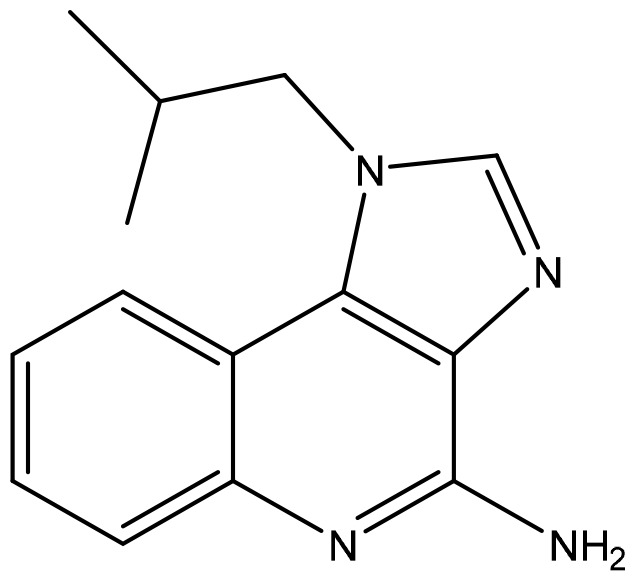
|
3M Pharmaceuticals | Marketed |
| TLR8 | Motolimod |
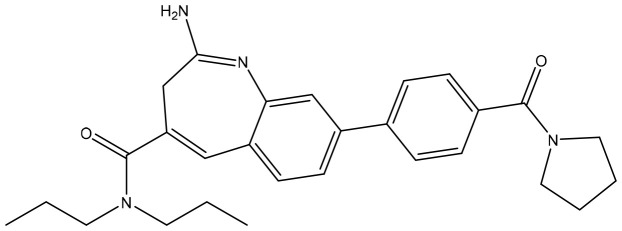
|
Array Pharma, Celgene | Phase I/II (NCT02431559) |
| ARG | INCB001158 |
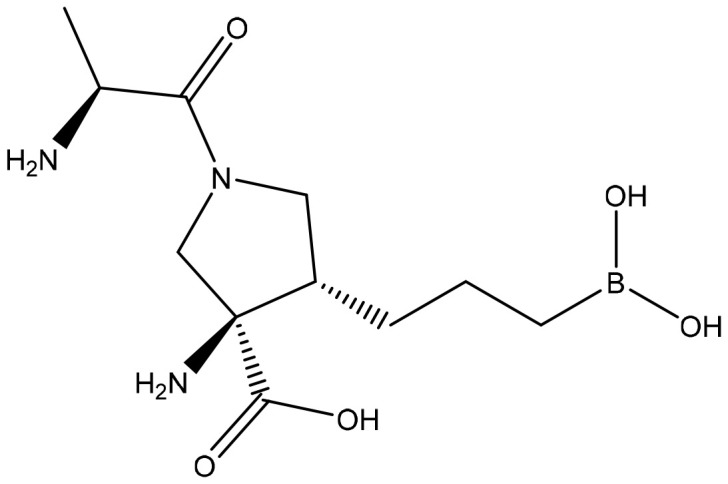
|
Calithera Biosciences, Incyte | Phase I/II (NCT02903914) |
