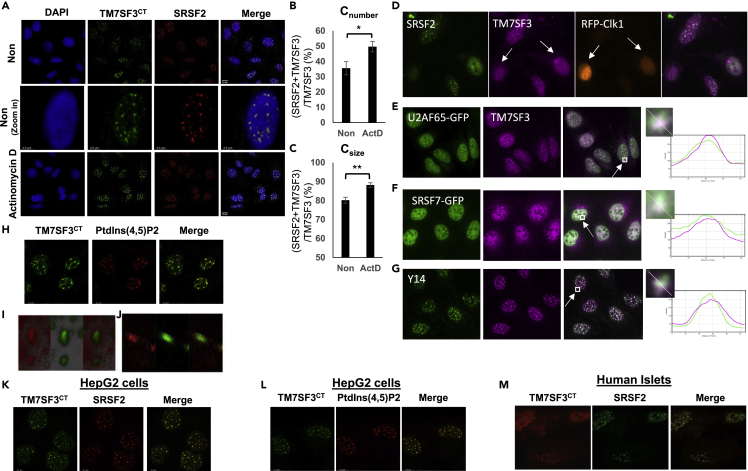
Figure 2.
TM7SF3 localizes to nuclear speckles
(A) U2-OS cells were treated with or without Actinomycin D (5 ug/mL) for 4 h. Cells were then stained with DAPI (blue) and immunostained with anti-TM7SF3CT (Green) or anti-SFRS2 (Red) antibodies.
(B) Cnumber is the percentage of the number of objects immunostained with both TM7SF3CT and anti-SFRS2 antibodies, over the total staining with TM7SF3CT antibodies. Results are presented as means ± SEM (∗p<0.05; ∗∗p<0.01).
(C) Csize is the size-based co-localization coefficients of the objects stained with TM7SF3CT and anti-SFRS2 antibodies over the total staining with TM7SF3CT antibodies.
(D) U2OS cells overexpressing RFP-Clk1 (red), marked by arrows, were immunostained with anti-SRSF2 (green) or anti-TM7SF3CT (magenta).
(E and F) U2OS cells stably overexpressing U2AF65-GFP (E) or SRSF7-GFP (F) (green) were immunostained with anti-TM7SF3CT (magenta).
(G) U2OS cells were stained with anti-Y14 (green) or anti-TM7SF3CT (magenta). The nucleus (E-G) was stained with Hoechst (blue). The intensity of immunoreactivity of specific regions, marked by squares, is shown on the right.
(H–J) co-staining of U2-OS cells with antibodies against PtdIns(4,5)P2 (red) and TM7SF3 (green; n = 2). A z stack 3D image (I) and A z stack section (J).
(K and L) HepG2 cells were stained with DAPI (blue), and immunostained with anti-TM7SF3CT (Green), anti-SFRS2 (Red) (K), or antibodies against PtdIns(4,5)P2 (red) and TM7SF3 (green) (L).
(M) Dispersed human islets were immunostained as described in K. Experiments were carried out at least three times unless otherwise indicated.