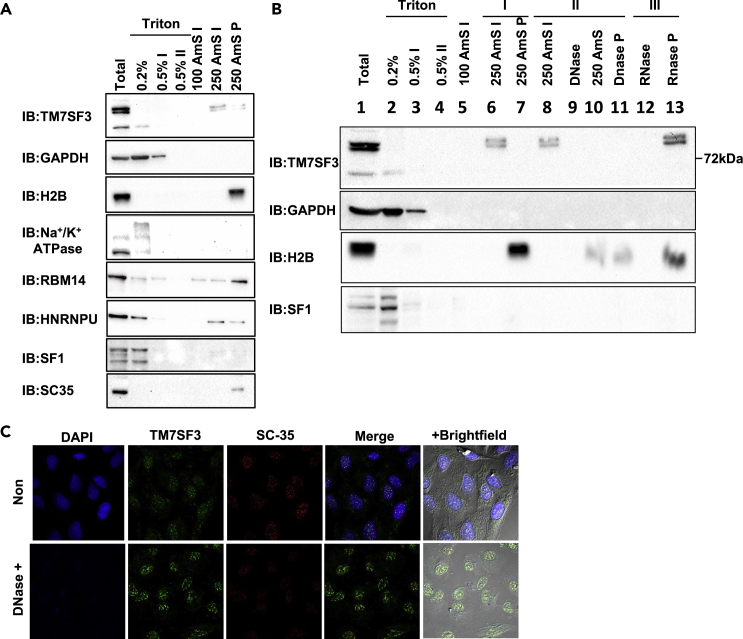
Figure 3.
Subcellular fractionation and localization of TM7SF3
(A) U2-OS cells were resuspended in 0.2% Triton X-100, centrifuged and the supernatants were collected as 0.2% Triton fraction. The pellets were resuspended in 0.5% Triton X-100; centrifuged and the supernatant was collected as 0.5% Triton fractions. The pellets were resuspended with the indicated concentrations of ammonium sulfate (AMS); centrifuged and the supernatant was collected as AMS fractions. The pellets were then suspended in ‘sample buffer’ (AMS P fractions). Samples were resolved by SDS-PAGE and immunoblotted with the indicated antibodies.
(B) HFF cells were extracted in ‘sample buffer’ (1). Alternatively, the cells were sequentially incubated with the indicated solutions [0.2% (2)→ 0.5% (3)→ 0.5% (II) (4) Triton X-100→ 100 mM AMS (5)→ 250 mM AMS (6)]. The supernatants of the indicated fractions (2–6) and the 250 mM AMS pellets (7) were collected and incubated with sample buffer. Next, supernatants of the 250 mM AMS extracts (8) were treated with DNase, centrifuged and the sups were collected (9). The pellets were incubated again with AMS 250 mM, centrifuged, and supernatants were collected as AMS post-DNase fractionation (10). The pellets were incubated with ‘sample buffer’ (11) or treated with RNase and centrifuged. The supernatants (12) and pellets (13) were collected. All samples were resolved by SDS-PAGE and immunoblotted with the indicated antibodies n = 3.
(C) U2-OS cells were incubated with 0.5% Triton X-100, followed by incubation with DNase. Cells were then fixed; stained with DAPI (blue) and immunostained with anti-TM7SF3CT (Green) or anti-SFRS2 (Red) antibodies. n = 2.