Abstract
Coronary perforation is a potentially life-threatening complication of percutaneous coronary intervention (PCI). We studied incidence, outcomes and temporal trends following PCI-related coronary artery perforation (CAP).
Methods
Prospective systematic review and meta-analysis including meta-regression using MEDLINE and EMBASE to November 2020. We included ‘all-comer’ PCI cohorts including large PCI registries and randomised controlled trials and excluding registries or trials limited to PCI in high-risk populations such as chronic total occlusion PCI or cohorts treated only with atheroablative devices. Regression analysis and corresponding correlation coefficients were performed comparing perforation incidence, mortality rate, tamponade rate and the rate of Ellis III perforations against the midpoint (year) of data collection to determine if a significant temporal relationship was present.
Results
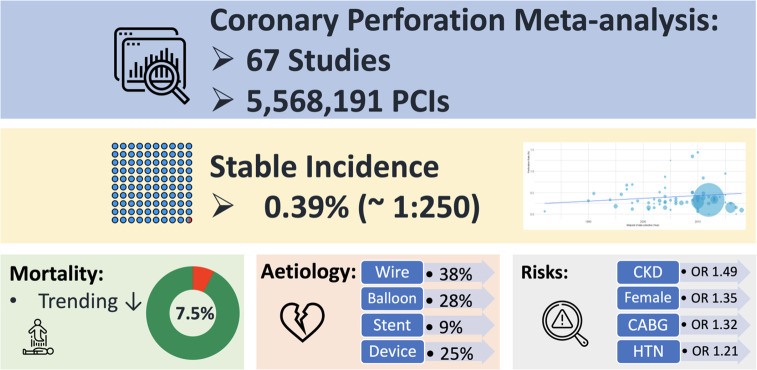
3997 studies were screened for inclusion. 67 studies met eligibility criteria with a total of 5 568 191 PCIs included over a 38-year period (1982–2020). The overall pooled incidence of perforation was 0.39% (95% CI 0.34% to 0.45%) and remained similar throughout the study period. Around 1 in 5 coronary perforations led to tamponade (21.1%). Ellis III perforations are increasing in frequency and account for 43% of all perforations. Perforation mortality has trended lower over the years (7.5%; 95% CI 6.7% to 8.4%). Perforation risk factors derived using meta-regression were female sex, hypertension, chronic kidney disease and previous coronary bypass grafting. Coronary perforation was most frequently caused by distal wire exit (37%) followed by balloon dilation catheters (28%). Covered stents were used to treat 25% of perforations, with emergency cardiac surgery needed in 17%.
Conclusion
Coronary perforation complicates approximately 1 in 250 PCIs. Ellis III perforations are increasing in incidence although it is unclear whether this is due to reporting bias. Despite this, the overall perforation mortality rate (7.5%) has trended lower in recent years. Limitations of our findings include bias that may be introduced through analysis of multidesign studies and registries without pre-specified standardised perforation reporting CMore research into coronary perforation management including the optimal use of covered stents seems warranted.
PROSPERO registration number
CRD42020207881.
Keywords: percutaneous coronary intervention, coronary artery disease
WHAT IS ALREADY KNOWN ON THIS TOPIC
The incidence of coronary perforation during contemporary percutaneous coronary intervention (PCI) varies according to studied population. Historically, the estimated incidence of coronary perforation in all-comer PCI is 0.43% based on previous pooled analysis. The relevance of this to contemporary practice is unknown as interventional cardiologists treat more complex patient subgroups. The aetiology, success of treatment modalities, outcomes and clinical risk factors for coronary perforation during PCI have had variable reporting within the literature.
WHAT THIS STUDY ADDS
Incidence of coronary perforation is stable over the last 40 years and occurs in ~ 1in 250 PCI procedures. Ellis III perforation are increasingly common with contemporary PCI but associated mortality with this feared complication is declining. We highlight a gender divide with women being at higher risk of coronary perforation during PCI. Patients with chronic kidney disease, prior coronary bypass grafting and hypertension were also at higher risk of coronary perforation during PCI.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Pooled, real-world, data coronary perforation during PCI helps cardiologists glean more information on procedural risk, guiding patients with treatment options and associated risks.
Introduction
Coronary artery perforation (CAP) is a potentially lethal complication of percutaneous coronary intervention (PCI) with incidence directly proportional to procedural complexity.1 PCI is increasingly used to treat complex calcified coronary anatomy which has been demonstrated to have a higher risk of periprocedural adverse events including perforation.2 The reported incidence of perforation during contemporary PCI varies broadly according to population studied but a historical pooled meta-analysis of 197 061 patients estimates incidence at 0.43% (95% CI 0.35% to 0.52%).3 Data from large PCI registries help cardiologists glean more information on procedural risk which helps guide patients with treatment options.4–7 Pooling data from these large databases can help us accurately estimate the overall incidence of coronary perforation during contemporary PCI.8
The primary objective of our study was to estimate overall incidence of CAP during PCI. We aimed to perform a comprehensive systematic review of CAP to study aetiology, treatment and clinical outcomes. Finally, we studied whether perforation incidence has changed in recent years hypothesising that an increased incidence is plausible as we treat increasingly complex lesions.
Methods
Coronary perforation incidence and temporal trends is a systematic review and meta-analysis performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.9 A comprehensive protocol was prospectively submitted and registered on The International Prospective Register of Systematic reviews prior to commencing the study or analysis.
Search strategy
A literature search of MEDLINE and EMBASE using the OVID interface was performed. Keywords used were ‘coronary artery’,
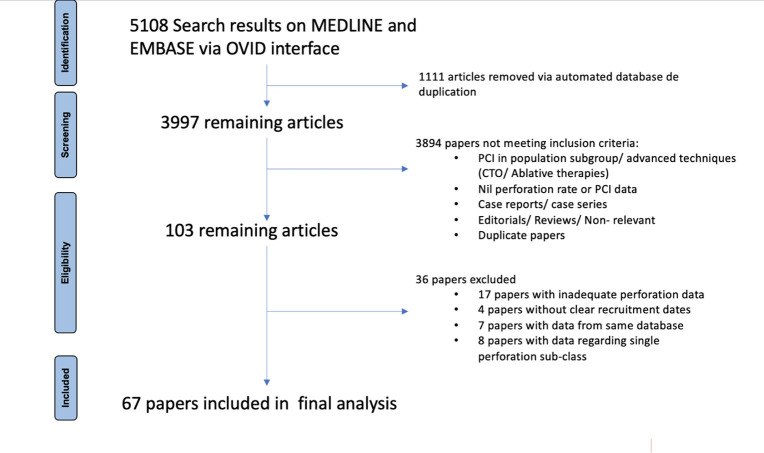
‘percutaneous coronary intervention’ and ‘perforation’ including their subheadings and synonyms. Results were restricted to articles available in English and pertaining to humans. The search was performed in September of 2020 yielding 5108 results which was reduced to 3997 articles after automatic de-duplication by the OVID interface. Titles and abstracts were reviewed for the pre-specified inclusion criteria by two investigators (PMik and CS). Discrepancies over eligibility and final inclusion were determined by a third investigator (TF).
Inclusion and exclusion criteria
For inclusion in this meta-analysis, it was essential for an article to report the number of perforations within the cohort of patients undergoing PCI. A range of time during which these procedures were performed was essential to allow for assessment of temporal trends. Any article not meeting these criteria was excluded from the analysis. Case reports and case series were excluded. However, abstracts presented at conferences were included to allow us to capture the reported incidence of CAP in real-world practice. Studies or datasets solely reporting results in higher risk patient cohorts (eg, chronic total occlusion (CTO) PCI or atheroablative devices) or non-routine procedural practice were excluded.
Data extraction
Data were extracted manually by two investigators (NH, MMon) and checked for accuracy by a third investigator (AB). Collected data included first author, year of publication, median year of patient recruitment, name of registry/trial and perforation rate. The population demographics of the studied population as well as the population who had CAP (mean age, sex, cardiovascular risk factors) were tabulated. Data pertaining to perforation severity (according to the Ellis criteria), cause, outcomes and management were also collected if available. If several articles had data from the same registry/trial, preference for inclusion in the final analysis was given to the article which had been peer reviewed and with the largest cohort of studied patients to reduce duplicate data from impacting final results. If unable to differentiate based on these parameters, a fourth investigator (PMik) would select between the papers with the article providing most data regarding our secondary objectives being selected for inclusion. Sixty-seven papers were included in the final statistical analysis4–7 10–72 (figure 1). Efforts to exclude data pertaining to CTO procedures within a larger dataset were made by the authors.
Figure 1.
Flow diagram of study selection according to the PRISMA guidelines. CTO, chronic total occlusion; PCI, percutaneous coronary intervention; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Definitions
CAP rate (%) was defined as (Total number of perforations/Total number of procedures)×100. The total number of non-CTO perforations was assumed to be equivalent to total number of perforations if the paper did not specify. Similarly, the total number of non-CTO procedures was assumed to be equivalent to total number of procedures if the paper did not specify the number of CTO procedures within their cohort. The total number of procedures was also assumed to be equal to the total number of patients within the cohort unless otherwise specified within the article. Perforation mortality rate (%) was calculated as (Total number of perforation-related deaths/Total number of perforations)×100, where perforation-related death has occurred during the procedure or within the acute phase post-procedure. Mortality of patients in the medium–long term after perforation (>30 days) was not analysed.
Statistical analysis
R Core Team V.4.0.3 was used to perform regression analyses and to calculate pooled estimates for proportions. Regression analysis was performed comparing perforation rate against the midpoint (year) of data collection to determine if a significant temporal relationship was present. Similar temporal analyses were performed for tamponade rates and rates of Ellis III perforation. Corresponding correlation coefficients were then calculated for these relationships. Perforation rates from included studies were plotted against time on a bubble plot, where bubble radius was scaled in proportion to study size. A sensitivity analysis was performed and plotted using studies that had a total data collection period of 7 years or less, in order to minimise bias from studies that collected data over long periods of time.
Meta-analysis of proportions was conducted using the ‘meta’ and ‘metafor’ packages in R V.4.0.3. Perforation rates were pooled using the inverse variance method. A random effects model was chosen for this analysis, as we assumed that perforation rates varied significantly between studies. This model allows us to assume a mean distribution of perforation rates across all included studies, rather than a fixed difference in effect size. Logit-transformed proportions were used for the summary measure to minimise the risk of skewed data at extreme ranges.73 Knapp-Hartung adjustments were applied to the random effects model to account for uncertainty in our estimation of between-study heterogeneity.74 75 Proportions were pooled for rates of overall perforation, perforation mortality, perforation tamponade and Ellis III perforation. Perforation causes (ie, balloon, stent, wire or device) and perforation treatment strategies (ie, medical management, balloon occlusion, surgery or covered stent) were also meta-analysed across studies. Where information was available, perforations were also stratified by vessel territory, and a subgroup analysis was performed to determine the mortality rate of Ellis III perforations specifically. We also aimed to evaluate whether specific baseline patient characteristics could predict coronary perforation. To achieve this, we calculated perforation ORs from studies where baseline characteristics were reported for both perforation and non-perforation groups. ORs were then pooled using the exact Mantel-Haenszel method using R V.4.0.3 statistical software with ‘meta’ and ‘metafor’ packages.76
Results
Perforation rate and vascular territory
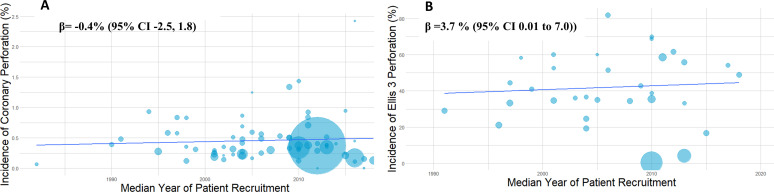
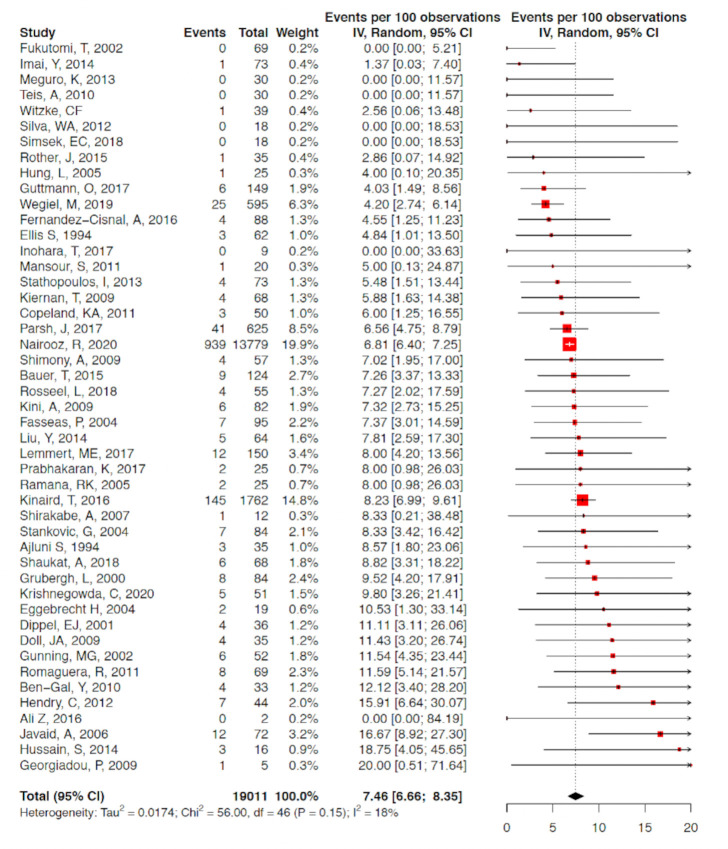
A total of 67 studies with 5 563 136 patients undergoing 5 568 191 procedures were eligible for inclusion in the analysis. A total of 19 776 coronary perforations were identified providing an estimated mean weighted perforation incidence of 0.39% (95% CI 0.34% to 0.45%) (figure 2). There were no significant temporal trends of perforation incidence with time (β=−0.4% (95% CI −2.5% to 1.8%), p=0.72) (figure 3A). A subgroup analysis of studies with recruitment periods of less than 7 years was performed to reduce the impact of studies with prolonged recruitment periods which again showed no significant correlation between perforation incidence over time (β=0.72% (95% CI −0.86% to 2.30%). The incidence of Ellis III perforation was reported in 29 studies (n=1455). Ellis grade III perforation accounted for 43.0% of perforations in those studies (95% CI 36.8% to 49.4%) (online supplemental appendix figure 1). The incidence of Ellis grade III perforation increased over time (β=3.7% (95% CI 0.01% to 7.0%), p=0.0494) (figure 3B).
Figure 2.
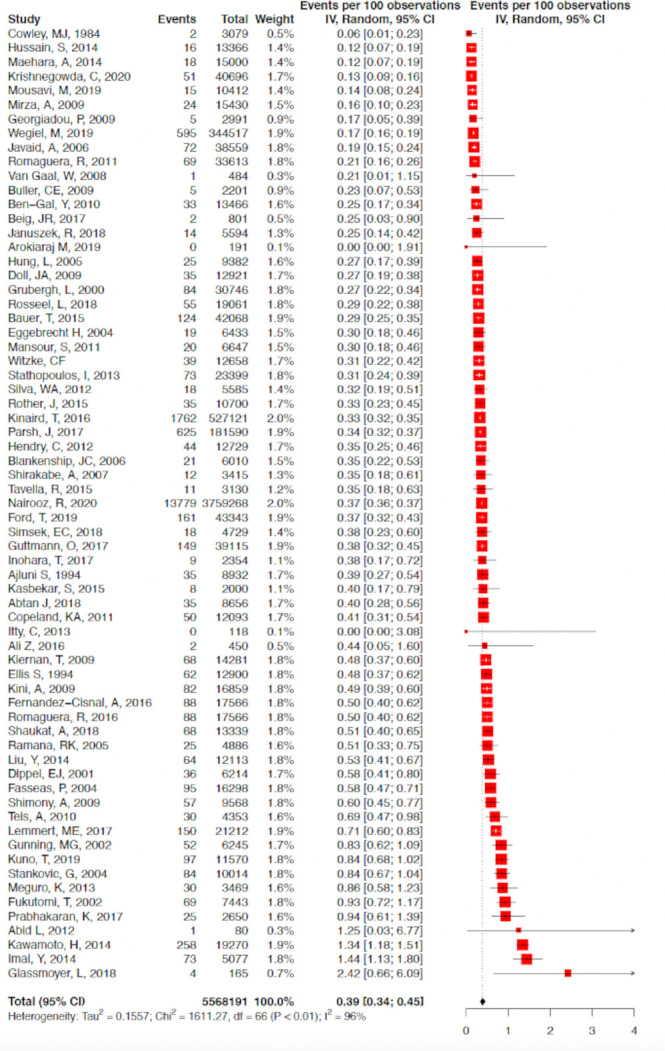
Forest plot of included studies reported coronary perforation in ‘all comer’ PCI. Data presented as % with 95% CI. PCI, percutaneous coronary intervention.
Figure 3.
(A) Temporal trend of coronary perforation. Bubble plot illustrating stable incidence of coronary perforation over the last four decades. Sample size of study represented by bubble size. (B) Temporal trend of Ellis grade III perforation. Bubble plot illustrating increasing incidence of Ellis grade III perforation over the last three decades. Sample size of study represented by bubble size.
openhrt-2022-002076supp001.pdf (14.4MB, pdf)
The site of coronary perforation was reported in 28 studies (n=4397). The left anterior descending (LAD), including Left Main Coronary artery (LMCA) were commonly affected (40.3% of perforations (95% CI 32.8% to 48.3%). 54.2% of perforations occurred in the left circumflex artery or Right coronary artery and only 6% of reported perforations occurred within a coronary bypass graft.
Outcomes of CAP
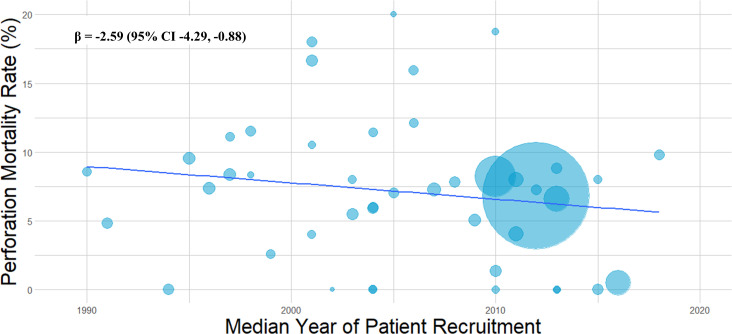
Perforation-associated mortality was determined from a pooled analysis of 47 studies (n=19 011). Perforation mortality was calculated at 7.5% (95% CI 6.7% to 8.4%) (figure 4). Notably, there was an overall decline in mortality related to CAP over time (β=−2.59% (95% CI −4.29% to –0.88%) p=0.0038) (figure 5).
Figure 4.
Forest plot of included studies reporting periprocedural mortality due to coronary perforation. Data presented as % with 95% CI.
Figure 5.
Temporal trend of perforation associated mortality. Bubble plot illustrating incidence of perforation associated mortality over the last three decades. Sample size of study represented by bubble size.
Forty-four studies (n=18 373) met pre-specified inclusion criteria to estimate incidence of cardiac tamponade due to CAP. Cardiac tamponade was observed in 21.1% of CAP (95% CI 17.2% to 25.8%) (online supplemental appendix figure 2a). The incidence of cardiac tamponade did not change over time (online supplemental appendix figure 2b).
Predictors of coronary perforation
Studies meeting pre-specified inclusion criteria were analysed to determine predictors of coronary perforation. Chronic kidney disease (CKD) (OR 1.49 (95% CI 1.11 to 1.98)), female gender (OR 1.35 (95% CI 1.30 to 1.41)), prior coronary bypass grafting (CABG) (OR 1.32 (95% CI 1.12 to 1.55)) and hypertension (OR 1.21 (95% CI 1.07 to 1.37)) were most strongly associated with coronary perforation. However, patients presenting with acute coronary syndromes appeared less likely to suffer from coronary perforation although the significance of this is uncertain (OR 0.88 (95% CI 0.66 to 1.19)). There was little impact on incidence of coronary perforation for patients with diabetes or hypercholesterolemia. Interventions on the left main/LAD coronary artery were associated with a modest increase in CAP (a summary of these findings is provided in table 1). Regression analysis did not demonstrate a correlation between age and incidence of coronary perforation (β=0.015 (95% CI −0.033 to 0.063) p=0.52) (online supplemental appendix figure 3).
Table 1.
Predictors of coronary artery perforation
| OR | 95% CI | |
| Female sex* | 1.35 | 1.30 to 1.41 |
| Previous CABG* | 1.32 | 1.12 to 1.55 |
| Chronic kidney disease† | 1.49 | 1.11 to 1.98 |
| Acute coronary syndrome‡ | 0.88 | 0.66 to 1.19 |
| Hypertension* | 1.21 | 1.07 to 1.37 |
| Diabetes* | 0.95 | 0.85 to 1.07 |
| Dyslipidaemia§ | 1.10 | 0.91 to 1.34 |
| Left main/LAD¶ | 1.16 | 1.04 to 1.31 |
Data presented as OR with 95% CI.
*Eight studies met pre-specified inclusion criteria (n=4 551 906).
†Three studies met pre-specified inclusion criteria (n=574 774).
‡Six studies met pre-specified inclusion criteria (n=4 528 971).
§Six studies met pre specified inclusion criteria (n=779 717).
¶Four studies met pre-specified inclusion criteria (n=597 709).
CABG, coronary bypass grafting; LAD, left anterior descending.
Coronary perforation aetiology
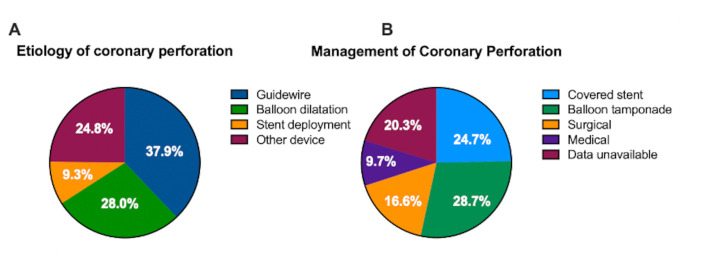
Twenty-nine studies met pre-specified inclusion criteria reporting aetiology of coronary perforation (n=1242). Coronary guidewires were the most frequent cause of coronary perforation during PCI accounting for 37.3% of reported perforations (95% CI 26.7% to 49.2%). Balloon dilatation pre and post-stent deployment accounted for 27.5% of perforations (95% CI 21.5% to 34.5%) with stent deployment accounting for 24.4% of CAP (95% CI 18.0% to 32.1%) and other devices causing 9.1% of perforations (95% CI 5.5% to 14.8%) (figure 6A).
Figure 6.
(A) Aetiology of coronary perforation from an analysis of 29 studies (n=1242). Pie graph illustrating the aetiology of coronary perforation. (B) Management of coronary perforation from an analysis of 39 studies (n=3258). Pie graph illustrating the definitive management option for coronary perforation. Data were inconclusive or unavailable in 20% of cases. Of the available data, coronary perforation was managed with covered stents for 31% of cases, balloon tamponade in 36% of cases, surgically in 21% of cases and medically in 12% of cases.
Management of coronary perforations
Thirty-nine studies met pre-specified criteria reporting management of coronary perforations (n=3258). Surgical management was required in 16.6% of patients with CAP (95% CI 10.94% to 24.4%). Approximately half of coronary perforations were able to be managed percutaneously with balloon tamponade (28.7% (95% CI 17.2% to 43.7%)) or use of a covered stent (24.7% (95% CI 14.7% to 38.6%). Conservative management was successfully used as the only treatment in 9.7% of perforations (95% CI 5.2% to 17.4%) without percutaneous or surgical intervention. The final treatment modality for CAP was indeterminate in 20.3% of analysed patients which prevented further analysis in this subgroup (figure 6B). Of the cases where the final treatment modality was available, coronary perforation was managed with covered stents for 31% of cases, balloon tamponade in 36% of cases, surgically in 21% of cases and medically in 12% of cases.
Discussion
This detailed systematic review provides the largest comprehensive overview of patients with PCI-related CAP. We demonstrate that (1) coronary perforation occurs in approximately 1 in 250 ‘all-comer’ PCI procedures, (2) the overall incidence is fairly steady but catastrophic perforations (Ellis III) are more common in recent years, (3) perforation mortality is fairly low but not insignificant (7.5%) and has declined over time, (4) female sex, kidney disease, previous CABG, hypertension and LAD target vessel are important clinical risk factors for perforation. Finally, (5) most coronary perforations are successfully managed with balloon tamponade or covered stents without requirement for surgical intervention figure 7.
Figure 7.
Central illustration. A summary of the major findings of COPIT. CABG, coronary bypass grafting; CKD, chronic kidney disease; COPIT, coronary perforation incidence and temporal trends; HTN, hypertension; PCI, percutaneous coronary intervention.
Perforation trends
As a community, we are tackling increasingly complex lesion subsets combined with an ageing population, hence it is certainly plausible that the rates of coronary perforation are higher now than in the formative years of PCI. Indeed, Kinnaird et al’s large UK study of coronary perforations using the BCIS database demonstrated there was a non-significant trend to higher perforation rates with time.5 The observation that perforation mortality has declined is reassuring and may reflect better recognition and ongoing education incorporating algorithmic management.1 We have demonstrated that Ellis III perforations are more common in recent years. Although not the subject of our research, this may be due to increased complex PCI with use of atheroablative devices, as well as contemporary trends for high-pressure post-dilation with 1:1 vessel sizing determined from diameter measured from external elastic membrane (EEM) to EEM.77 It is also plausible that there is increased recognition and use of the Ellis criteria since its introduction in 1994.25
Predictors of coronary perforation
Risk of CAP was found to be highest in distinct subgroups: females, patients with CKD, patients with hypertension and patients with previous CABG and LAD target vessel. Older age is a known robust predictor of adverse events with PCI,5 however, without individual patient data, studying the effects of age as a risk factor for perforation is an important limitation of this study level meta-analysis. This relates to large heterogeneity in age and its effects on outcomes depending on its inclusion as a continuous or categorical variable between studies. The higher incidence of coronary calcification and complex lesions are likely to explain the elevated risk of coronary perforation during PCI in patients with CKD and prior CABG. The reason for increased incidence of CAP in women remains unclear and is worthy of consideration. Female sex has consistently been shown as a predictor of adverse outcomes with PCI, including perforation.78 Smaller vessel sizes in females may be a relevant contributor. Additionally, arterial remodelling creating stiffer vessels due to changes in vascular smooth muscle leading to reduced vessel compliance is likely contributory.79 80 Patients with CKD were also found to be at higher risk of CAP during PCI, contributing to the body of evidence suggesting judicious use of PCI in this population subgroup.81 In patients with coronary artery bypass grafts, it is unclear from the available data whether these perforations occurred during interventions on graft or native vessels. The trend towards a lower rate of coronary perforation in patients presenting with acute coronary syndromes may reflect the younger age, the likelihood of a simpler PCI strategy and potentially less high-pressure stent optimisation during post-dilatation.
Unsurprisingly, cardiac tamponade is a common sequela of coronary perforation occurring in approximately 1 in 5 patients with incidence remaining stable. Perforation is a potentially lethal condition with a point estimate of mortality at approximately 7.5%, translating as 1 in 13 patients dying in the periprocedural period. We noted an overall decline in mortality over the last decade and the reasons for this may be multifactorial. Increased education and training on how to manage large perforations with use of ‘ping-pong’ guide catheters, introduction of covered stents with subsequent iterative development of increasingly deliverable devices and improved system recognition may all be relevant.82 In the present meta-analysis, approximately 25% of coronary perforations were managed with a covered stent but we hypothesise that in the contemporary treatment of catastrophic Ellis III perforation this figure is likely to be much higher despite the elevated risk of stent thrombosis after implantation. Despite incomplete data, our analysis would suggest that 85%–90% of CAP can be managed successfully without surgical intervention. It is worth noting that the use of covered stents has not completely negated the need for surgical salvage in patients with CAP. This may be relevant in CTO PCI with rare cases of dry tamponade resulting from perforation particularly in patients with prior CABG.
Limitations
Our meta-analysis using study level data has several important limitations. First, the analysis is limited by the available data and is subject to publication bias as well as subjective interpretation of presence of coronary perforations and their severity by investigators and operators which have not been standardised. Second, the data regarding management of coronary perforations is confounded by the likelihood of multiple management modalities that may be combined, hence determining the definitive successful perforation treatment strategy is imperfect. Third, a proportion of patients within the analysed data may have had CTO procedures despite the efforts of the investigators to remove these cases from the analysis. Fourth, many studies had to be excluded from secondary endpoint analyses including perforation aetiology and risk factors due to stringent criteria with lack of available data on clinical risk factors provided in most manuscripts. Fifth, we noted Ellis III perforation incidence increased with time, however, this subgroup analysis should be interpreted with caution particularly given the limited number of studies reporting incidence of Ellis III perforation. Finally, we performed multiple analyses that were pre-specified, however, we did not carry out testing for multiplicity, hence subgroup analyses should be considered hypothesis generating.
Conclusion
Coronary perforation is a recognised complication of PCI with an incidence of around 1 in 250 procedures (0.39%). Severe perforations (Ellis III) have become increasingly common however overall perforation mortality rate (7.5%) has trended lower in recent years. Female sex, CKD, hypertension or a history of CABG are common clinical risk factors for coronary perforation. More research into coronary perforation prevention and management strategies are warranted.
Acknowledgments
We acknowledge the study authors and patients who participated in these registries and clinical trials.
Footnotes
Twitter: @amorrow90, @tomjford
Contributors: The above work is the combined work of the authors. The study was devised by TF. The search was performed by PMik. Title, abstract and full text filtering was performed by PMik and CS. Data extraction was performed by NH and MMon. The data were checked for accuracy by AB. Statistical analysis was performed by PJ. PMcC, CA, AM, DC, MMcE were involved in manuscript design, edit and critical review. Study design and oversight by TF and PMik who are guarantors of the work including management of data, study conduct and decision to publish.
Funding: COPIT is an investigator-initiated study performed without external funding.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Lemmert ME, van Bommel RJ, Diletti R, et al. Clinical characteristics and management of coronary artery perforations: a single-center 11-year experience and practical overview. J Am Heart Assoc 2017;6:e007049. 10.1161/JAHA.117.007049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stone GW, Généreux P, Harrington RA, et al. Impact of lesion complexity on peri-procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: core laboratory analysis from 10 854 patients from the CHAMPION PHOENIX trial. Eur Heart J 2018;39:4112–21. 10.1093/eurheartj/ehy562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimony A, Joseph L, Mottillo S, et al. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol 2011;27:843–50. 10.1016/j.cjca.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 4. Nairooz R, Parzynski CS, Curtis JP, et al. Contemporary trends, predictors and outcomes of perforation during percutaneous coronary intervention (from the NCDR Cath PCI registry). Am J Cardiol 2020;130:37–45. 10.1016/j.amjcard.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 5. Kinnaird T, Kwok CS, Kontopantelis E, et al. Incidence, determinants, and outcomes of coronary perforation during percutaneous coronary intervention in the United Kingdom between 2006 and 2013: an analysis of 527 121 cases from the British cardiovascular intervention Society database. Circ Cardiovasc Interv 2016;9:e003449. 10.1161/CIRCINTERVENTIONS.115.003449 [DOI] [PubMed] [Google Scholar]
- 6. Parsh J, Seth M, Green J, et al. Coronary artery perforations after contemporary percutaneous coronary interventions: evaluation of incidence, risk factors, outcomes, and predictors of mortality. Catheter Cardiovasc Interv 2017;89:966–73. 10.1002/ccd.26917 [DOI] [PubMed] [Google Scholar]
- 7. Rakowski T, Węgiel M, Siudak Z, et al. Prevalence and predictors of coronary artery perforation during percutaneous coronary interventions (from the ORPKI national registry in Poland). Am J Cardiol 2019;124:1186–9. 10.1016/j.amjcard.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 8. Weintraub WS. Role of big data in cardiovascular research. J Am Heart Assoc 2019;8:e012791. 10.1161/JAHA.119.012791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abid L, Abdennadher M, Frikha Z, et al. 075 immediate and long term outcomes of the percutaneous coronary intervention of the ostia of the major epicardial coronary arteries. Archives of Cardiovascular Diseases Supplements 2012;4:24. 10.1016/S1878-6480(12)70471-8 [DOI] [Google Scholar]
- 11. Abtan J, Sorbets E, Popovic B, et al. P5106Prevalence, clinical characteristics and outcomes of procedural complications of percutaneous coronary intervention in non ST-elevation myocardial infarction: insights from the TAO trial. Eur Heart J 2018;39:1062. 10.1093/eurheartj/ehy566.P5106 [DOI] [Google Scholar]
- 12. Ajluni SC, Glazier S, Blankenship L, et al. Perforations after percutaneous coronary interventions: clinical, angiographic, and therapeutic observations. Cathet Cardiovasc Diagn 1994;32:206–12. 10.1002/ccd.1810320303 [DOI] [PubMed] [Google Scholar]
- 13. Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: optimize PCI): a randomised controlled trial. Lancet 2016;388:2618–28. 10.1016/S0140-6736(16)31922-5 [DOI] [PubMed] [Google Scholar]
- 14. Arokiaraj MC. Angioplasty with stenting in acute coronary syndromes with very low contrast volume using 6F diagnostic catheters and bench testing of catheters. Open Access Maced J Med Sci 2019;7:1004–12. 10.3889/oamjms.2019.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauer T, Boeder N, Nef HM, et al. Fate of patients with coronary perforation complicating percutaneous coronary intervention (from the Euro heart survey percutaneous coronary intervention registry). Am J Cardiol 2015;116:1363–7. 10.1016/j.amjcard.2015.07.056 [DOI] [PubMed] [Google Scholar]
- 16. Beig JR, Shah TR, Hafeez I, et al. Clinico-angiographic profile and procedural outcomes in patients undergoing percutaneous coronary interventions: the Srinagar registry. Indian Heart J 2017;69:589–96. 10.1016/j.ihj.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ben-Gal Y, Weisz G, Collins MB, et al. Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv 2010;75:708–12. 10.1002/ccd.22331 [DOI] [PubMed] [Google Scholar]
- 18. Blankenship JC, Haldis T, Feit F, et al. Angiographic adverse events, creatine kinase-MB elevation, and ischemic end points complicating percutaneous coronary intervention (a REPLACE-2 substudy). Am J Cardiol 2006;97:1591–6. 10.1016/j.amjcard.2005.12.050 [DOI] [PubMed] [Google Scholar]
- 19. Buller CE, Rankin JM, Carere RG, et al. Percutaneous coronary intervention in the occluded artery trial: procedural success, hazard, and outcomes over 5 years. Am Heart J 2009;158:408–15. 10.1016/j.ahj.2009.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Copeland KA, Hopkins JT, Weintraub WS, et al. Long-term follow-up of polytetrafluoroethylene-covered stents implanted during percutaneous coronary intervention for management of acute coronary perforation. Catheter Cardiovasc Interv 2012;80:53–7. 10.1002/ccd.23339 [DOI] [PubMed] [Google Scholar]
- 21. Cowley MJ, Dorros G, Kelsey SF, et al. Acute coronary events associated with percutaneous transluminal coronary angioplasty. Am J Cardiol 1984;53:12C–16. 10.1016/0002-9149(84)90738-0 [DOI] [PubMed] [Google Scholar]
- 22. Dippel EJ, Kereiakes DJ, Tramuta DA, et al. Coronary perforation during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade: an algorithm for percutaneous management. Catheter Cardiovasc Interv 2001;52:279–86. 10.1002/ccd.1065 [DOI] [PubMed] [Google Scholar]
- 23. Doll JA, Nikolsky E, Stone GW, et al. Outcomes of patients with coronary artery perforation complicating percutaneous coronary intervention and correlations with the type of adjunctive antithrombotic therapy: pooled analysis from REPLACE-2, acuity, and HORIZONS-AMI trials. J Interv Cardiol 2009;22:453–9. 10.1111/j.1540-8183.2009.00494.x [DOI] [PubMed] [Google Scholar]
- 24. Eggebrecht H, Ritzel A, von Birgelen C, et al. Acute and long-term outcome after coronary artery perforation during percutaneous coronary interventions. Z Kardiol 2004;93:791–8. 10.1007/s00392-004-0123-z [DOI] [PubMed] [Google Scholar]
- 25. Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era. incidence, classification, management, and outcome. Circulation 1994;90:2725–30. 10.1161/01.cir.90.6.2725 [DOI] [PubMed] [Google Scholar]
- 26. Fasseas P, Orford JL, Panetta CJ, et al. Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J 2004;147:140–5. 10.1016/S0002-8703(03)00505-2 [DOI] [PubMed] [Google Scholar]
- 27. Fernández-Cisnal A, Romaguera R, Ñato M, et al. Temporal trends in frequency, management and outcomes of coronary perforations. Minerva Cardioangiol 2018;66:361–7. 10.23736/S0026-4725.18.04433-X [DOI] [PubMed] [Google Scholar]
- 28. Ford TJ, Adamson C, Morrow AJ, et al. Coronary artery perforations: Glasgow natural history study of covered stent coronary interventions (GNOCCI) study. J Am Heart Assoc 2022;74:B248. 10.1161/JAHA.121.024492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukutomi T, Suzuki T, Popma JJ, et al. Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J 2002;66:349–56. 10.1253/circj.66.349 [DOI] [PubMed] [Google Scholar]
- 30. Georgiadou P, Karavolias G, Sbarouni E, et al. Coronary artery perforation in patients undergoing percutaneous coronary intervention: a single-centre report. Acute Card Care 2009;11:216–21. 10.1080/17482940903254207 [DOI] [PubMed] [Google Scholar]
- 31. Glassmoyer L, Walters D, Almasoud A. Percutaneous coronary intervention for coronary artery bypass graft turndowns in contemporary practice: characteristics and outcomes. Catheterization and Cardiovascular Interventions 2018;91:S62–3. [Google Scholar]
- 32. Gruberg L, Pinnow E, Flood R, et al. Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol 2000;86:680–2. a8. 10.1016/s0002-9149(00)01053-5 [DOI] [PubMed] [Google Scholar]
- 33. Gunning MG, Williams IL, Jewitt DE, et al. Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart 2002;88:495–8. 10.1136/heart.88.5.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guttmann OP, Jones DA, Gulati A, et al. Prevalence and outcomes of coronary artery perforation during percutaneous coronary intervention. EuroIntervention 2017;13:e595–601. 10.4244/EIJ-D-16-01038 [DOI] [PubMed] [Google Scholar]
- 35. Hendry C, Fraser D, Eichhofer J, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention 2012;8:79–86. 10.4244/EIJV8I1A13 [DOI] [PubMed] [Google Scholar]
- 36. Huang CM, Young MS, Jen HL. Single-Center experience with transradial coronary intervention. Acta Cardiologica Sinica 2002;18:51–60. [Google Scholar]
- 37. Hussain S, Kayani AM, Munir R. Description and follow-up of patients with coronary perforations during percutaneous coronary interventions at AFIC-NIHD. J Coll Physicians Surg Pak 2014;24:290–2. doi:04.2014/JCPSP.290292 [PubMed] [Google Scholar]
- 38. Imai Y, Tarutani Y, Hosaka F, et al. TCT-126 thrombin clot injection for the treatment of coronary wire perforation. J Am Coll Cardiol 2014;64:B38. 10.1016/j.jacc.2014.07.157 [DOI] [Google Scholar]
- 39. Inohara T, Numasawa Y, Higashi T, et al. Predictors of high cost after percutaneous coronary intervention: a review from Japanese multicenter registry overviewing the influence of procedural complications. Am Heart J 2017;194:61–72. 10.1016/j.ahj.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 40. Itty C, Blenkhorn A, Mumford D, et al. Outcomes of percutaneous coronary intervention without onsite cardiac surgery in a new regional cardiac catheter lab in northern New South Wales. Heart, Lung and Circulation 2013;22:S146–7. 10.1016/j.hlc.2013.05.349 [DOI] [Google Scholar]
- 41. Januszek R, Dziewierz A, Siudak Z, et al. Chronic obstructive pulmonary disease and periprocedural complications in patients undergoing percutaneous coronary interventions. PLoS One 2018;13:e0204257. 10.1371/journal.pone.0204257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Javaid A, Buch AN, Satler LF, et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol 2006;98:911–4. 10.1016/j.amjcard.2006.04.032 [DOI] [PubMed] [Google Scholar]
- 43. Kasbekar S, Tilak S, Gautam N. Impact of monitoring of Cath lab quality indicators. Indian Heart J 2015;67:S41. 10.1016/j.ihj.2015.10.100 26995429 [DOI] [Google Scholar]
- 44. Kawamoto H, Tanaka K, Takagi K. Long-Term clinical outcomes after polytetrafluoroethylene-covered stent implantation for coronary perforation. European Heart Journal 2014;35:192. [DOI] [PubMed] [Google Scholar]
- 45. Kiernan TJ, Yan BP, Ruggiero N, et al. Coronary artery perforations in the contemporary interventional era. J Interv Cardiol 2009;22:350–3. 10.1111/j.1540-8183.2009.00469.x [DOI] [PubMed] [Google Scholar]
- 46. Kini AS, Rafael OC, Sarkar K, et al. Changing outcomes and treatment strategies for wire induced coronary perforations in the era of bivalirudin use. Catheter Cardiovasc Interv 2009;74:700–7. 10.1002/ccd.22112 [DOI] [PubMed] [Google Scholar]
- 47. Krishnegowda C, Puttegowda B, Krishnappa S, et al. "Incidence, clinical and angiographic characteristics, management and outcomes of coronary artery perforation at a high volume cardiac care center during percutaneous coronary intervention". Indian Heart J 2020;72:232–8. 10.1016/j.ihj.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuno T, Sawano M, Numasawa Y. Use of intravascular ultrasound in the modern percutaneous coronary intervention: a report from a Japanese multicenter registry. European Heart Journal 2016;37:866.27462685 [Google Scholar]
- 49. Lemmert ME, Van Bommel RJ, Diletti R. Daemen J and van Mieghem nm. coronary artery perforation-a single center experience. European Heart Journal 2017;38:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y, Xu Z, Peng W, et al. Study of coronary artery perforation during percutaneous coronary intervention in the Cangzhou Chinese population. Acta Cardiol 2014;69:139–43. 10.1080/ac.69.2.3017294 [DOI] [PubMed] [Google Scholar]
- 51. Maehara A, Mintz GS, Bui AB, et al. Intravascular ultrasound evidence of perivascular trauma during routine percutaneous coronary intervention. Int J Cardiovasc Imaging 2014;30:849–56. 10.1007/s10554-014-0413-0 [DOI] [PubMed] [Google Scholar]
- 52. Mansour S, Matteau A, Noiseux N, et al. 719 incidence, predictors and long-term clinical outcomes of coronary perforation in the modern era. Can J Cardiol 2011;27:S327. 10.1016/j.cjca.2011.07.594 [DOI] [Google Scholar]
- 53. Meguro K, Ohira H, Nishikido T, et al. Outcome of prolonged balloon inflation for the management of coronary perforation. J Cardiol 2013;61:206–9. 10.1016/j.jjcc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 54. Mirza AJ, Taha AY, Aldoori JS, et al. Coronary artery perforation complicating percutaneous coronary intervention. Asian Cardiovasc Thorac Ann 2018;26:101–6. 10.1177/0218492318755182 [DOI] [PubMed] [Google Scholar]
- 55. Mousavi M, Poorhosseini H, Nematipour E, et al. Effect of age on procedural success, complications, and clinical outcome from a large angioplasty registry. Crit Pathw Cardiol 2019;18:23–31. 10.1097/HPC.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 56. Prabhakaran KK, Koshy G, Iype M, et al. Coronary perforation-a tertiary center experience. Eur Heart J 2017;38:675. 10.1093/eurheartj/ehx504.P3300 26491108 [DOI] [Google Scholar]
- 57. Ramana RK, Arab D, Joyal D, et al. Coronary artery perforation during percutaneous coronary intervention: incidence and outcomes in the new interventional era. J Invasive Cardiol 2005;17:603–5. [PubMed] [Google Scholar]
- 58. Romaguera R, Sardi G, Laynez-Carnicero A, et al. Outcomes of coronary arterial perforations during percutaneous coronary intervention with bivalirudin anticoagulation. Am J Cardiol 2011;108:932–5. 10.1016/j.amjcard.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 59. Romaguera R, Fernández Cisnal A, Ñato M, et al. TCT-253 temporal trends in frequency, management and outcomes of coronary perforations. J Am Coll Cardiol 2016;68:B103. 10.1016/j.jacc.2016.09.381 [DOI] [PubMed] [Google Scholar]
- 60. Rosseel L, Scott B, Prihadi E, et al. Is a covered stent justifiable in the treatment of coronary artery perforation? An observational analysis of long-term results of two different covered stent types. Catheter Cardiovasc Interv 2019;93:419–25. 10.1002/ccd.27892 [DOI] [PubMed] [Google Scholar]
- 61. Röther J, Tröbs M, Ludwig J, et al. Treatment and outcome of coronary artery perforations using a dual guiding catheter technique. Int J Cardiol 2015;201:479–83. 10.1016/j.ijcard.2015.08.138 [DOI] [PubMed] [Google Scholar]
- 62. Shaukat A, Tajti P, Sandoval Y, et al. Incidence, predictors, management and outcomes of coronary perforations. Catheter Cardiovasc Interv 2019;93:48–56. 10.1002/ccd.27706 [DOI] [PubMed] [Google Scholar]
- 63. Shimony A, Zahger D, Van Straten M, et al. Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol 2009;104:1674–7. 10.1016/j.amjcard.2009.07.048 [DOI] [PubMed] [Google Scholar]
- 64. Shirakabe A, Takano H, Nakamura S, et al. Coronary perforation during percutaneous coronary intervention lessons from our experiences. Int Heart J 2007;48:1–9. 10.1536/ihj.48.1 [DOI] [PubMed] [Google Scholar]
- 65. Silva WA, Costa RA, Campostrini T, et al. Incidence, management and prognosis of coronary perforations. Revista Brasileira de Cardiologia Invasiva 2012;20:295–302. 10.1016/S2214-1235(15)30068-5 [DOI] [Google Scholar]
- 66. Simsek EC, Kırıs T, Emren SV. Management and outcomes of coronary artery perforation during routine percutaneous coronary intervention without advanced procedure: a single-centre report. Am J Cardiol 2018;121:e72–3. 10.1016/j.amjcard.2018.03.183 [DOI] [Google Scholar]
- 67. Stankovic G, Orlic D, Corvaja N, et al. Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations. Am J Cardiol 2004;93:213–6. 10.1016/j.amjcard.2003.09.042 [DOI] [PubMed] [Google Scholar]
- 68. Stathopoulos I, Kossidas K, Panagopoulos G, et al. Cardiac tamponade complicating coronary perforation during angioplasty: short-term outcomes and long-term survival. J Invasive Cardiol 2013;25:486–91. [PubMed] [Google Scholar]
- 69. Tavella R, Worthley M, Arstall M, et al. Contemporary percutaneous coronary intervention practice: assessment of procedure complications. Heart, Lung and Circulation 2015;24:S380. 10.1016/j.hlc.2015.06.623 [DOI] [Google Scholar]
- 70. Teis A, Teis-Soley A, Fernández-Nofrerías E, et al. Coronary artery perforation by intracoronary guide wires: risk factors and clinical outcomes. Rev Esp Cardiol 2010;63:730–4. 10.1016/s1885-5857(10)70148-1 [DOI] [PubMed] [Google Scholar]
- 71. van Gaal WJ, Arnold JR, Porto I, et al. Long term outcome of elective day case percutaneous coronary intervention in patients with stable angina. Int J Cardiol 2008;128:272–4. 10.1016/j.ijcard.2007.05.054 [DOI] [PubMed] [Google Scholar]
- 72. Witzke CF, Martin-Herrero F, Clarke SC, et al. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol 2004;16:297–301. [PubMed] [Google Scholar]
- 73. Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA, US: Sage Publications, Inc, 2001. [Google Scholar]
- 74. IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods 2019;10:83–98. 10.1002/jrsm.1316 [DOI] [PubMed] [Google Scholar]
- 76. Efthimiou O, Debray TPA, van Valkenhoef G, et al. GetReal in network meta-analysis: a review of the methodology. Res Synth Methods 2016;7:236–63. 10.1002/jrsm.1195 [DOI] [PubMed] [Google Scholar]
- 77. Zhang J, Gao X, Kan J, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol 2018;72:3126–37. 10.1016/j.jacc.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 78. Ford TJ, Khan A, Docherty KF, et al. Sex differences in procedural and clinical outcomes following rotational atherectomy. Catheter Cardiovasc Interv 2020;95:232–41. 10.1002/ccd.28373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mozos I, Maidana JP, Stoian D, et al. Gender differences of arterial stiffness and arterial age in smokers. Int J Environ Res Public Health 2017;14:565. 10.3390/ijerph14060565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Łoboz-Rudnicka M, Jaroch J, Kruszyńska E, et al. Gender-Related differences in the progression of carotid stiffness with age and in the influence of risk factors on carotid stiffness. Clin Interv Aging 2018;13:1183–91. 10.2147/CIA.S161711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bangalore S, Maron DJ, Fleg JL, et al. International study of comparative health effectiveness with medical and invasive Approaches-Chronic kidney disease (ISCHEMIA-CKD): rationale and design. Am Heart J 2018;205:42–52. 10.1016/j.ahj.2018.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. May A, Bhagwandeen R, Collins N. Contemporary management of coronary artery perforation. Heart Lung Circ 2019;28:e121–5. 10.1016/j.hlc.2019.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002076supp001.pdf (14.4MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.