Abstract
In this study we investigated the impact of parental language input on language development and associated neuroscillatory patterns in toddlers at risk of Autism Spectrum Disorder (ASD). Forty-six mother-toddler dyads at either high (n=22) or low (n=24) familial risk of ASD completed a longitudinal, prospective study including free-play, resting electroencephalography, and standardized language assessments. Input quantity/quality at 18 months positively predicted expressive language at 24 months, and relationships were stronger for high-risk toddlers. Moderated mediations revealed that input-language relationships were explained by 24-month frontal and temporal gamma power (30–50 Hz) for high-risk toddlers who would later develop ASD. Results suggest that high-risk toddlers may be cognitively and neurally more sensitive to their language environments, which has implications for early intervention.
Keywords: Autism, EEG, language development, language input, early experience
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by persistent deficits in social communication and social interaction across multiple contexts (American Psychiatric Association, 2013). However, children with ASD exhibit vast heterogeneity in the developmental trajectories and ultimate attainment of broader receptive and expressive language skills such as vocabulary and grammar (Tager-Flusberg, 2006; Tager-Flusberg et al., 2005). It is estimated that 75 percent of children diagnosed with ASD have some level of language impairment, including 30 percent who are minimally verbal (Anderson et al., 2007; Tager-Flusberg & Kasari, 2013), yet other children with ASD exhibit age-appropriate or even above-average language skills (Tager-Flusberg et al., 2011). Even younger siblings of affected children who will not develop ASD are at higher-than-average risk of language delay (Marrus et al., 2018). Given that early language skills in children with ASD predict later life outcomes, such as educational attainment and adult independence (Billstedt et al., 2005; Gotham et al., 2012; Howlin et al., 2004; Miller et al., 2017), it is critical to better understand the factors influencing, and mechanisms underlying, heterogeneity of language development within this population.
The Role of Language Experience in Autism
One exogenous factor associated with children’s language development is the language input they are exposed to from caregivers. In typically developing children, both the quantity (e.g., number of words) and quality (e.g., content of speech) of early language input predicts trajectories of language development across childhood (for review, see Rowe & Weisleder, 2020). Similar input-skill relationships are found both in children with ASD diagnoses as well as toddlers at high risk of developing ASD due to having an affected older sibling (henceforth referred to as “high risk toddlers”), and relationships follow expected developmental trajectories (for review, see Naigles, 2013; Swanson, 2020). For example, amongst children diagnosed with ASD and pre-diagnosis high-risk toddlers, better language development is predicted by the quantity of words heard in the first year of life (Swanson et al., 2019), the grammatical complexity of input in toddlerhood (Choi et al., 2020), the use of expansions in response to speech by preschool age children (Swensen et al., 2007), and the frequency of responsive, synchronized utterances across childhood (Siller & Sigman, 2002).
Alternatively, several studies have aimed to determine whether diagnosed/high-risk children may experience systematically different language input than typically developing children. These studies find little to no difference in the quantity of speech directed to children with/without ASD diagnoses (Bang & Nadig, 2015; Siller & Sigman, 2002; Warren et al., 2010) and with/without familial risk of ASD (Leezenbaum et al., 2014; Swanson et al., 2019; Talbott et al., 2016). In contrast, a few studies suggest that the language directed to children with ASD may be less grammatically complex than that directed to typically developing children (Choi et al., 2020; Fusaroli et al., 2019). However, it is critical to note that this research does not suggest that the quantity and/or quality of caregiver speech in any way increases the risk for the child developing autism. Rather, it is likely that child-level developmental differences that are already under way invite quantitatively and qualitatively different input, which leads to reciprocal influences between caregiver and child (Bottema-Beutel & Kim, 2020; Tager-Flusberg, 2016). Instead, these findings highlight the importance of supporting caregiver interaction in children already at risk of developmental disorders to help prevent further language delay or impairment (Swanson, 2020).
Neurodevelopment of Language in Autism
Inquiry into the mechanisms underlying the vast heterogeneity in language development in both typically and atypically developing children is guided by the rapid neurodevelopment of language systems early in life (Skeide & Friederici, 2016). Electroencephalography (EEG) is a non-invasive method of measuring brain activity well-suited for infants and toddlers (Nelson & McCleery, 2008; Saby & Marshall, 2012; Xie & Nelson, 2021). While many EEG studies have examined brain responses to linguistic stimuli (see Friederici, 2005 for review), brain oscillations in the absence of language provide a unique window into neural function underlying language development (Benitez-Burraco & Murphy, 2019). Oscillations reflect synchronized fluctuations in neuronal firing, which facilitates the development of efficient neural networks (Uhlhaas et al., 2010), and are grouped by the frequencies of fluctuations. The gamma band, which includes frequencies from 30–50 Hz, signifies a balance of excitation and inhibition that regulates experience-dependent neuroplasticity (Levin & Nelson, 2015). Power in the gamma band rapidly increases in the first year of life (Gabard-Durnam et al., 2019; Pivik et al., 2018; Pivik et al., 2019), peaks between 3–5 years of age, particularly over frontal regions (Takano & Ogawa, 1998), and decreases thereafter (Tierney et al., 2013). Thus, higher gamma power in infancy and early childhood may indicate neural maturity and processes that promote efficient cognitive development (Anderson & Perone, 2018; Gou et al., 2011).
Resting (or baseline) gamma power has been associated with linguistic and cognitive development across early childhood. In typically developing children, higher frontal gamma power from 16–36 months is associated with higher concurrent and later language skills (Benasich et al., 2008; Gou et al., 2011), and frontal gamma power in newborns also positively predicts language scores at 15 months of age (Tarullo et al., 2012). Additionally, frontal and parietal gamma power in socioeconomically disadvantaged infants and children is associated with receptive language skills as well as nonlinguistic cognitive skills such as memory and executive functioning (Brito et al., 2016; Tarullo et al., 2017). Further, left-central gamma power in infants mediates the well-established effect of socioeconomic status on language skills (Cantiani et al., 2019). Together, these results suggest that gamma-band oscillations may reflect synchronization in developing language networks (Tomalski et al., 2013) and support attunement to and integration of the perceptual components of language (Csibra et al., 2000; Fries, 2009; Ortiz-Mantilla et al., 2016).
Gamma dysregulation has also been implicated in language development in children with/at risk for ASD. Atypical evoked gamma activity has been found during linguistic processing in children with ASD (Kolesnik et al., 2019; Ortiz-Mantilla et al., 2019), and gamma fluctuations during speech processing is correlated with verbal scores in adults (Jochaut et al., 2015). Further abnormalities are seen in EEG at rest. For example, in the same prospective study from which the current data are drawn, high-risk infants (ages 3 and 6 months) exhibit lower baseline gamma power than low-risk infants (Levin et al., 2017; Tierney et al., 2012), and reduced gamma power growth predicts later ASD diagnoses (Gabard-Durnam et al., 2019), which may indicate insufficient neural inhibition and atypical neuroplasticity. Also from this study, baseline frontal gamma power in high-risk two-year-olds was negatively associated with concurrent expressive language scores (i.e., lower gamma power predicts better language scores; Wilkinson et al., 2019). Similar reverse relationships have been documented between gamma power and measures of overall developmental delay (Orekhova et al., 2007) and verbal impairment (Jochaut et al., 2015). This suggests that the neuroscillatory underpinnings of language and cognitive development may differ in children at risk of and/or diagnosed with ASD (Wilkinson et al., 2020).
The Present Study
This study aims to integrate findings regarding input-driven language development and the neurophysiological mechanisms underlying language development. Several recent studies using magnetic resonance imaging have begun to identify functional (King et al., 2021; Romeo, Leonard, et al., 2018) and structural (Merz et al., 2020; Romeo et al., 2020; Romeo, Segaran, et al., 2018) brain mechanisms linking characteristics of caregiver language input to language development in typically developing infants and children. Additionally, recent EEG studies with socioeconomically disadvantaged infants have found that greater quantities of language input in the first year of life is related to greater gamma power as well as lower frequency bands (Brito et al., 2020; Pierce et al., 2020). However, it is currently unknown whether these neural mechanisms may differ in children with ASD, who may be disproportionately sensitive to their early language environments.
The present study addresses this gap by investigating whether the neuroscillatory mechanisms that longitudinally link toddlers’ language input to their receptive and expressive language skills vary by ASD risk and/or diagnosis. We specifically focus on development from 18–24 months, because it is prior to diagnosis and because input during this window has been found to have the strongest effects on long-term language outcomes (Gilkerson et al., 2018). Furthermore, we investigate gamma power over both frontal and temporal regions as potential mechanisms, given the importance of both regions for receptive and expressive language and their rapid development in early life (Skeide & Friederici, 2016). As illustrated in Figure 1, we hypothesize that (1) resting gamma power in language-related frontal and temporal regions will mediate the relationship between toddlers’ language input and their language development, (2) risk status and diagnostic outcome will moderate the direct relationship between input and language development, with affected toddlers showing stronger input-language relationships, and (3) familial risk and diagnostic outcome will moderate the indirect relationship through frontotemporal gamma power, which would indicate that the neural mechanisms for experience-dependent language development differ according to ASD risk/diagnosis.
Figure 1.
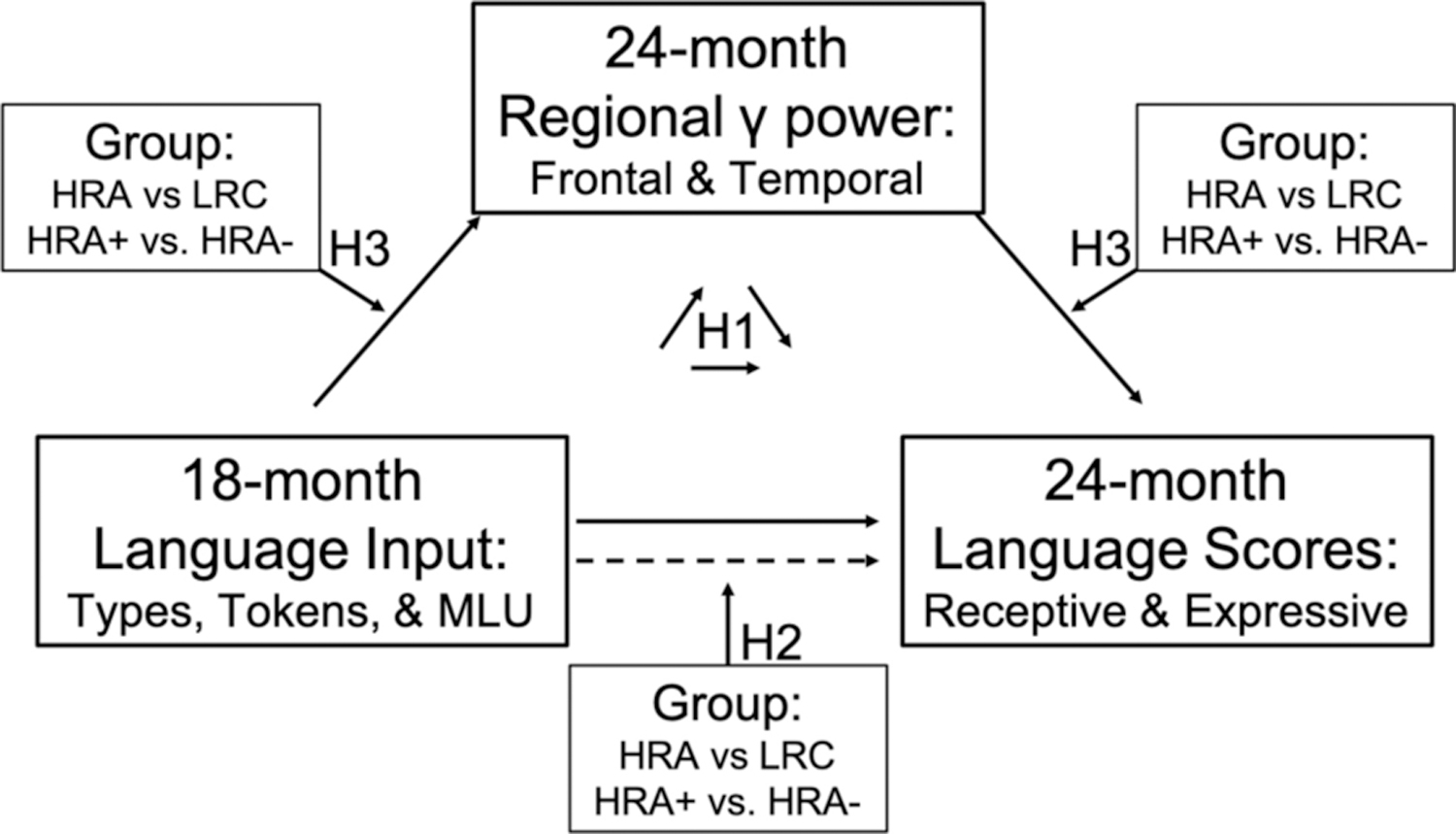
Conceptual figure representing the tested moderated mediation model and associated hypotheses. Hypothesis 1 (H1) addresses the mediation, hypothesis 2 (H2) addresses the moderation of the direct effect, and hypothesis 3 (H3) addresses the moderation of the indirect effect. MLU= Mean Length of Utterance.
Methods
Participants
The present study included a subsample of 46 mother-toddler dyads (23 male) drawn from a larger prospective, longitudinal study of 3–36-month-old children at high or low risk of developing ASD by virtue of having an older sibling with autism (for full sample description, see Gabard-Durnam et al., 2019; Wilkinson et al., 2020). Because younger siblings of children with ASD are more likely to develop an autism spectrum disorder than the general public (estimated to be as high as 1 in 5, Messinger et al., 2015; Ozonoff et al., 2011), so-called baby-sibling designs provide enriched samples for prospectively studying early ASD markers. Participants were recruited at or before 12-months of age into one of two groups: infants at high risk of ASD (HRA, n=22) had an older sibling with a community diagnosis of ASD that could not be attributed to a known genetic disorder, while low risk controls (LRC, n=24) had a typically developing older sibling and no first- or second-degree family history of ASD. Older sibling diagnosis was confirmed independently using the Social Communication Questionnaire (SCQ; Rutter et al., 2003) and/or the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). At their final visit to the lab, children received a final ASD diagnosis using both the ADOS and best clinical judgment by a Licensed Clinical Psychologist. Within the present sample, all LRC children were determined to not have ASD, 10 HRA children were determined to have ASD (HRA+), and 12 HRA children were determined to not have ASD (HRA−). LRC and HRA children did not differ on participant sex, caregiver education, family income, race, or ethnicity (Table 1). HRA+ and HRA− children did not differ on caregiver education, family income, race, or ethnicity, but they did differ on sex (HRA+ included more males, p = 0.04). No independent or dependent variables were significantly associated with caregiver education, family income, race, or ethnicity, so no covariates were included in analyses. The lack of these associations is not surprising given the limited demographic variability within the sample, which is later discussed as a limitation.
Table 1.
Sample Characteristics
| Measure | LRC | HRA (all) | HRA− | HRA+ | LRC v. HRA | HRA+ v. HRA− |
|---|---|---|---|---|---|---|
| N | 24 | 22 | 12 | 10 | ||
| Sex | 13M, 11F | 10M, 12F | 3M, 9F | 7M, 3 F | n.s. | p = .04 |
| Race | n.s. | n.s. | ||||
| White | 21 | 17 | 10 | 7 | ||
| Black/African American | 1 | 0 | 0 | 0 | ||
| Asian | 1 | 0 | 0 | 0 | ||
| Multiple Races | 1 | 5 | 2 | 3 | ||
| Ethnicity | n.s. | n.s. | ||||
| Hispanic/Latino | 0 | 1 | 0 | 1 | ||
| Not Hispanic/Latino | 24 | 21 | 12 | 9 | ||
| Parental Education (parent with highest education level) | n.s. | n.s. | ||||
| Less than 4-year college degree | 1 | 5 | 2 | 3 | ||
| 4-year college degree | 6 | 7 | 2 | 5 | ||
| Advanced degree | 14 | 9 | 7 | 2 | ||
| Unknown | 3 | 1 | 1 | 0 | ||
| Annual Household Income | n.s. | n.s. | ||||
| Less than $75,000 | 4 | 2 | 0 | 2 | ||
| Greater than $75,000 | 16 | 18 | 11 | 7 | ||
| Unknown | 4 | 2 | 1 | 1 | ||
| Language Input Measures (per 10 minutes) | ||||||
| Tokens (quantity) | 625.2 ± 185.0 | 579.3 ± 162.8 | 574.9 ± 144.0 | 584.5 ± 190.9 | n.s. | n.s. |
| Types (diversity) | 168.1 ± 44.1 | 157.9 ± 35.1 | 162.8 ± 38.1 | 152.1 ± 32.0 | n.s. | n.s. |
| MLU (complexity) | 3.49 ± 0.50 | 3.06 ± 0.50 | 3.21 ± 0.57 | 2.89 ± 0.36 | p = .006 | n.s. |
| 24-month Scores on the Mullen Scales of Early Learning (MSEL) | ||||||
| Receptive Language | 59.7 ± 7.7 | 52.4 ± 11.5 | 52.8 ± 9.5 | 51.8 ± 14.0 | p = .02 | n.s. |
| Expressive Language | 59.1 ± 10.5 | 48.9 ± 8.7 | 50.8 ± 5.9 | 46.5 ± 11.0 | p < .001 | n.s. |
| Verbal Developmental Quotient | 118.0 ± 16.1 | 101.7 ± 15.5 | 104.2 ± 10.3 | 98.8 ± 20.3 | p = .001 | n.s. |
Note: LRC = low risk control; HRA = high risk of Autism; HRA+ = high risk of Autism, diagnosed with ASD; HRA− = high risk of Autism, not diagnosed with ASD; n.s. = not significant. Categorical variables are reported as N per group, while continuous variables are reported as mean ± standard deviation. Between-group p-values come from Fisher’s Exact test (gender, income), Mann-Whitney U-test (parental education), or Welch’s t-test (all continuous variables).
Inclusion criteria required infants to have a minimum gestational age of 36 weeks, no history of pre- or postnatal medical or neurological problems, and no known genetic disorders (e.g., fragile-X, tuberous sclerosis). The complete study enrolled 255 infants. Seventeen infants from the LRC group were excluded after enrollment for not meeting inclusion criteria (4 had a family history of ASD, 8 had an older sibling that met ADOS criteria for ASD, 4 revealed the qualifying older sibling was a half sibling, and 1 was diagnosed with hearing impairment), and 1 infant from the HRA group was excluded for developing seizures. Forty-seven participants discontinued the study before they could receive a final clinical judgement. Additionally, 3 from the LRC group were excluded for meeting criteria for ASD, and 5 from the HRA group were excluded for receiving a diagnosis other than ASD (e.g., language impairment). This left 182 children in the larger longitudinal study.
For determining the subsample for the present analyses, 9 participants were excluded for not hearing English as their primary language at home (>80% of time). At the 18-month-old visit, 69 participants did not have usable parent-child free play videos for coding language input due to session non-attendance (n=8), task non-completion (n=31), or file corruption (n=30). Of the remaining 104 participants, a pseudo-random sample of 70 videos (oversampled for HRA+) were selected for transcription (see Choi et al., 2020). At the 24-month visit, 16 children did not attend, 7 did not have usable language assessment data due to task non-completion, and 45 did not have usable baseline EEG data (n = 24 not acquired, 6 incomplete recordings, 1 technical error, and 14 poor quality data, see below), leaving 103 participants with both EEG and language assessment data. The 46 toddlers in the present sample comprised all participants with usable data from all three measures, including 18-month parent-child interaction, 24-month baseline EEG, and 24-month standardized language scores.
Language Input Measures
At 18-months of age, parent-child dyads engaged in video-recorded free play in the laboratory for 10 minutes. Families were provided with a set of age-appropriate toddler toys, and parents were asked to play with their child as they normally would. Videotaped sessions were transcribed verbatim following Codes for the Human Analysis of Transcripts (CHAT) conventions of the Child Language Data Exchange System (CHILDES; MacWhinney, 2000). All parent and child verbalizations were transcribed at the utterance level, which were demarcated by a pause, a change in conversational turn, or a change in intonational pattern. Trained transcribers were determined to have met reliability when they achieved 95% agreement on utterance boundaries. Additionally, each transcript was verified by a second transcriber to ensure accuracy and consistency.
Transcripts were automatically analyzed in Computerized Language ANalysis (CLAN; MacWhinney, 2000), and three measures of parent input were extracted: (1) Tokens indicates the number of total words spoken by the parent and indexed input quantity, (2) Types indicates the number of unique words spoken by the parent and indexed vocabulary diversity, and (3) Mean length of utterance (MLU) indicates the average number of morphemes per utterance and indexes grammatical complexity. While tokens are exclusively a quantitative measure of input, types and MLU index more qualitative aspects of the input.
Child Language Skill Measures
At 24 months of age, toddlers were administered the Mullen Scales of Early Learning (MSEL; Mullen, 1995), a standardized assessment of physical, cognitive, and verbal skills. Receptive and expressive language skills were separately indexed by the age-normed T-scores from the Receptive Language Scale and the Expressive Language Scale.
ASD outcome classification
At their final visit to the lab (24 or 36 months of age), children received a final ASD diagnosis. Research staff with extensive experience administered and scored the ADOS, and an ADOS-reliable researcher co-scored via video recording. When children met or came within three points of the ASD cutoff score on the ADOS, a Licensed Clinical Psychologist reviewed scores and assessment videos to provide a best estimate clinical judgment using DSM-5 criteria. Children from either group who were determined to have a disorder other than ASD (e.g., attention deficit disorder, anxiety, developmental language disorder) were excluded. The majority of children (n=42) had their diagnostic outcomes determined at 36 months; however, because of sample attrition, 4 children had their ASD outcomes determined at 24 months (1 HRA+, 2 HRA−, and 1 LRC). These four children were not statistical outliers within their group on any independent or dependent measure, and their inclusion is supported by evidence of the diagnostic stability of ASD in high-risk children between 18 and 36 months (Ozonoff et al., 2015; Zwaigenbaum et al., 2016).
EEG Data Acquisition
At 24 months of age, 2–5 minutes of continuous baseline/resting EEG data were acquired while toddlers were seated on their caregivers’ laps in a dimly lit, sound-attenuated, electrically shielded room, following field standards (deBoer et al., 2007). A research assistant sat nearby and ensured toddlers were calm and still by blowing bubbles and/or quietly showing toys but did not engage in social interaction. EEG data were collected using either a 128-channel (n=44) or 64-channel (n=2) Geodesic Sensor Net System, sampled at 250 or 500 Hz, and re-referenced online to the vertex (channel Cz) through NetStation software (Electrical Geodesics, Inc (EGI), Eugene, OR, USA). The study began with the 64-channel net but switched to 128-channel nets because the production company ceased supporting 64-channel net equipment. Thus, channels were selected for analysis from spatial locations that corresponded across nets (Gabard-Durnam et al., 2019). Impedances were kept below 100KΩ, in accordance with the capabilities of the high-impedance DC-coupled amplifiers (Net Amps 200 or Net Amps 300) inside the electrically shielded room.
EEG Processing
The continuous, baseline EEG portion of the raw NetStation files were exported to MATLAB (R2017a). Preprocessing, artifact removal, and quality assessment was conducted with the Harvard Automated Processing Pipeline for EEG (HAPPE), a preprocessing pipeline optimized for developmental EEG data with short recordings and/or high levels of artifact (Gabard-Durnam et al., 2018). All files were batch processed using the batch EEG automated processing platform (BEAPP) to ensure the same artifact removal criteria were applied regardless of group or acquisition circumstances (Levin et al., 2018). A whole-head distributed subset of channels was processed through HAPPE (128-channel net: 3, 4, 9, 11, 13, 19, 20, 22, 23, 24, 27, 28, 33, 36, 40, 41, 45, 46, 47, 52, 58, 62, 70, 75, 83, 92, 96, 98, 102, 103, 104, 108, 109, 112, 117, 118, 122, 123, 124; 64-channel net: 2, 3, 6, 8, 9, 11, 12, 13, 15, 16, 17, 21, 24, 25, 27, 28, 34, 37, 40, 46, 49, 50, 52, 53, 54, 57, 58, 61, 62). A 1-Hz digital high-pass filter and a 100-Hz low-pass filter were applied, and data sampled at 500 Hz were resampled to 250 Hz with interpolation as recommended for HAPPE processing.
HAPPE’s artifact identification and removal steps were applied, including removal of 60 Hz electrical noise through multi-taper approach, bad channel rejection, and participant-produced artifact rejection (e.g., eye blinks, movement, and muscle activity) through wavelet-enhanced ICA and multiple artifact rejection algorithm (MARA; Winkler et al., 2014). After artifact rejection, any channels removed were repopulated through spherical interpolation. Data were then re-referenced to the average reference and detrended using the signal mean. EEGs were then segmented into contiguous 2-second windows to allow for power calculations using multitaper spectral analysis (Babadi & Brown, 2014), and any segments with retained artifact were rejected using HAPPE’s amplitude and joint probability criteria. Noncontiguous data were not concatenated. The lengths of raw and/or processed EEG did not differ by recruitment or diagnostic outcome group (all p > 0.1).
EEG Rejection
All 24-month EEG files (n=148) were subjected to the same pre- and post-processing pipeline no matter whether participants were ultimately included in the final study sample. EEGs were rejected if they had fewer than 20 postprocessed good segments (40 seconds) or were more than 3 standard deviations from the mean on the following HAPPE metrics: percent good channels (<82%), mean retained artifact probability (>0.3), median retained artifact probability (>0.35), percent of independent components rejected as artifact (>84%), and percent of EEG signal variance retained after artifact removal (<32%), which resulted in the rejection of 8 EEGs. Additionally, EEGs with a mean power in any frequency band more two standard deviations from their outcome group mean were visually reviewed blind to outcome group status, which resulted in the rejection of 2 additional EEGs. Neither HAPPE quality measures nor visual inspection rejection rates differed by recruitment or diagnostic outcome group (all p > 0.1). Quality measures were also not correlated with power in the gamma band (p > 0.1), which is the EEG measure of interest in the current study.
EEG Power Decomposition
A Fast Fourier Transform with multitaper windowing (3 orthogonal tapers) was used to decompose the signal into power for each segment at each channel of interest. Total power in the gamma band (30–50 Hz) was calculated as the summed power across all frequencies within the band, equivalent to the area under the power density curve (Gabard-Durnam et al., 2019). Power at each channel was then averaged across all 2-second segments within the recording and normalized by a log base-10 transform. Finally, power was averaged across all channels within two regions of interest (ROIs): one centered over the frontal cortex (128-channel net: 3, 4, 11, 19, 20, 23, 24, 27, 118, 123, 124; 64-channel net: 2, 3, 8, 9, 12, 13, 58, 62), and one centered over bilateral temporal-parietal cortex (128: channel net—40, 41, 45, 46, 47, 52, 92, 98, 102, 103, 108, 109; 64-channel net: 21, 24, 25, 28, 46, 50, 52, 53; Supplementary Figure 1).
Statistical Analyses
Demographic differences between recruitment and diagnostic outcome groups were explored using Fisher’s exact test (gender and sex) and Mann-Whitney U-test (parental education, which was ordinal and non-normally distributed, see Table 1). Welch’s t-test was used to explore group differences in all measures of interest: language input, regional gamma power, and MSEL language scores. Pearson’s correlations were used to assess relationships between measures of interest within groups, and the effect of group (risk/diagnosis) on these relationships was examined through multiple regressions with group (dummy-coded) as an interaction term.
Mechanistic relationships between measures of interest were examined by estimating first-stage, second-stage, and combined first- and second-stage moderated mediation models (Edwards & Lambert, 2007; Hayes, 2015). Separate models were constructed to assess whether either regional gamma measure (frontal or temporal) mediated the association between each input measure (tokens, types, MLU) and each language measure (receptive and expressive scores), and further, whether the indirect effect was conditional on risk status (HRA or LRC, within the full sample) or diagnostic outcome (HRA+ or HRA−, within the HRA subgroup). All continuous measures were mean-centered prior to creating product terms, and the index of moderated mediation was tested with a with 95% bias-corrected bootstrap confidence interval based on 10,000 replications (Hayes, 2015). Statistical analyses were completed in RStudio using R v4.0, with exception of moderated mediation models, which were completed using PROCESS v3.5 (Hayes, 2018) executed in SPSS 26.
Results
Input, Language, and EEG Differences by Group
Table 1 and Figure 2 show comparisons between LRC and HRA groups on the four input measures and three language score measures. The only language input measure that differed by risk group was the mean length of utterance [t(43.57) = 2.91, p < .01], such that LRC toddlers were on averaged exposed to longer utterances (M = 3.49 morphemes) than HRA toddlers (M = 3.06 morphemes). LRC toddlers exhibited higher scores than HRA toddlers on both language measures, with larger differences in expressive language scores (EL: [t(43.51) = 3.62, p < .001], mean difference = 10.26 points) than receptive language scores (RL: [t(36.39) = 2.51, p < .05], mean difference = 7.30 points). HRA and LRC groups did not differ in either frontal or temporal gamma power. No input, EEG, or language score measure significantly differentiated HRA+ and HRA− groups.
Figure 2.
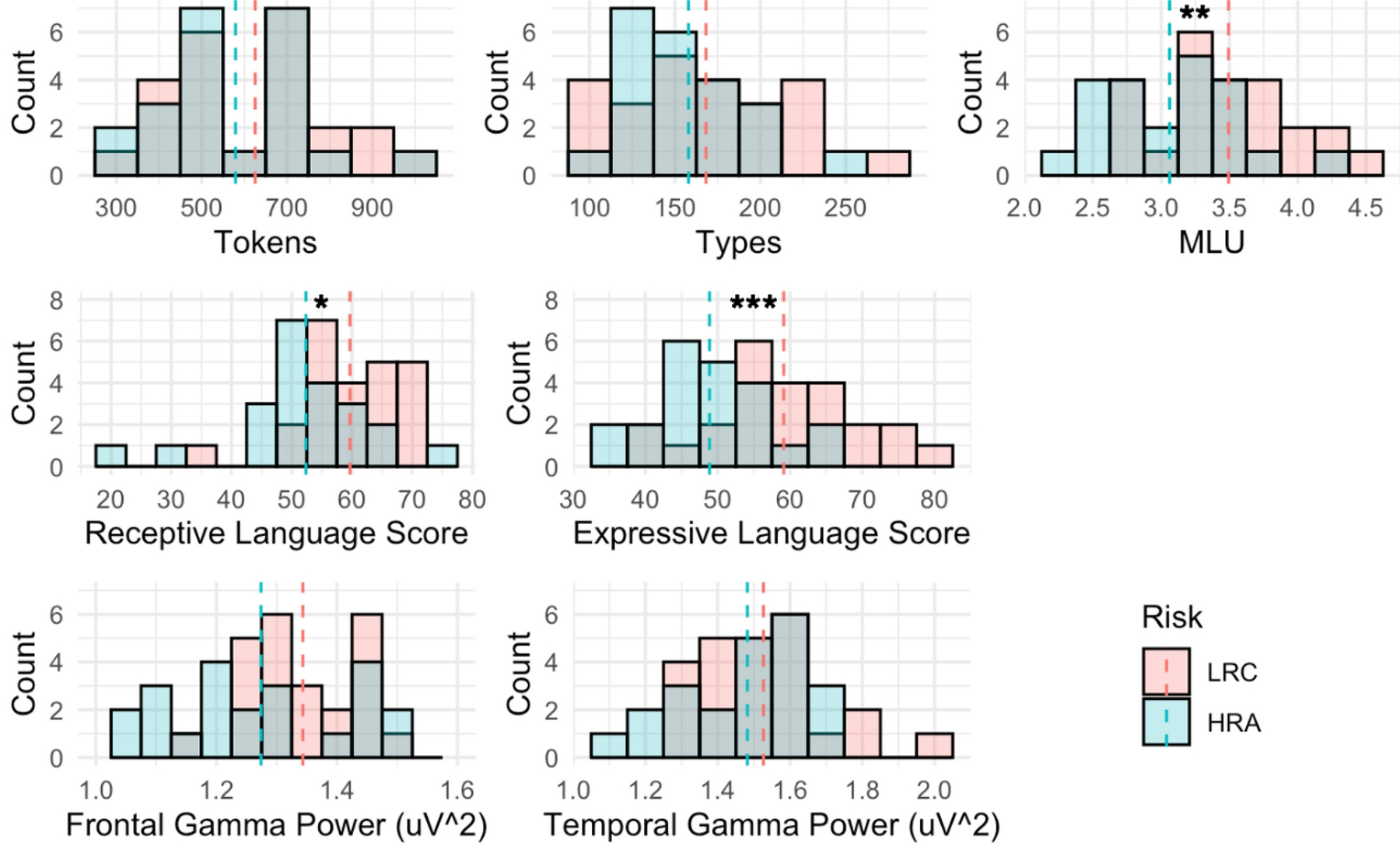
Distributions of primary measures by risk group. The top row displays language input measures; the middle row displays scores on the language assessments of the Mullen Scales of Early Learning; and the bottom row displays the log10-transformed absolute power in the gamma band (30–50 Hz) over frontal and temporal scalp regions. Group means are represented by dashed lines, and asterisks indicate significant between-group differences according to Welch’s t-test. MLU= Mean Length of Utterance; LRC = low risk control; HRA = high risk of Autism. *p < .05, **p < .01, ***p < .001
Relationships between Input and Language Scores
Within the full sample (collapsed across groups), parent word types at 18-months significantly predicted toddlers’ 24-month receptive language scores (r = .33, p < .05) and marginally predicted toddler’ 24-month expressive language scores (r = .27, p = .07), while parent MLU at 18-months significantly predicted both receptive and expressive language scores (receptive: r = .39, p < .01; expressive: r = .51, p < .001). Multiple regressions including all three input measures revealed that MLU was the only significant predictor of receptive (β = .31, p < .05) and expressive (β = .48, p < .01) language scores after controlling for the other measures.
For two of three input measures, relationships with expressive language were moderated by risk group (Tokens*Risk: β = 1.20, p < .05; Types*Risk: β = 1.17, p<.05; MLU*Risk: n.s.), such that Tokens and Types significantly predicted expressive language scores in HRA but not LRC toddlers (HRA: Tokens: r = .59, p < .01; Types: r = .63, p < .01; Figure 3, top row). MLU significantly predicted expressive language scores in HRA toddlers (r =.44, p < .05) and marginally in LRC toddlers (r =.37, p = .08), but the interaction with risk was not significant. No relationships between input measures and receptive language scores were significantly moderated by risk.
Figure 3.
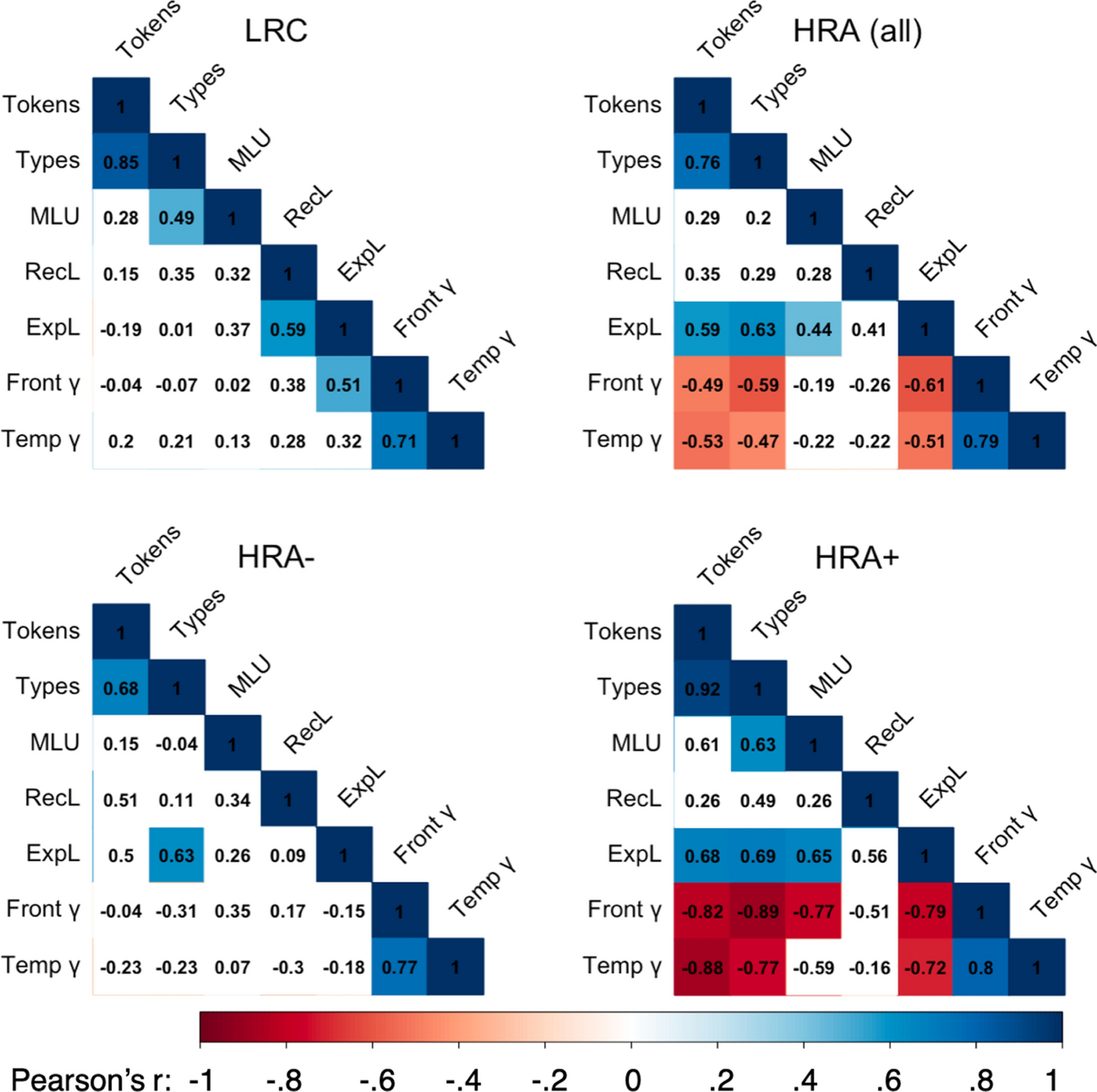
Correlation matrices of all independent, dependent, and mediator variables by risk group (top row) and diagnostic group within the high-risk group (bottom row). Text in cells represents the Pearson product-moment correlation coefficient, cool colors represent significant positive correlations, warm colors represent significant negative correlations, and white cells represent non-significant relationships. LRC = low risk control; HRA = high risk of Autism; HRA+ = High risk of Autism, diagnosed with ASD; HRA− = high risk of Autism, not diagnosed with ASD; MLU= Mean Length of Utterance; RecL = Receptive Language Score; ExpL = Expressive Language Score; Front γ = log10 transformed absolute power in the gamma band (30–50 Hz) over frontal scalp regions; Temp γ = log10 transformed absolute power in the gamma band (30–50 Hz) over temporal scalp regions.
Within the HRA group, diagnostic outcome (ASD versus no ASD) also moderated the relationship between input MLU and expressive language scores (β = 2.98, p < .05), such that MLU significantly predicted expressive language scores in HRA+ toddlers (r = .65, p < .05) but not HRA− toddlers (Figure 3, bottom row, and Figure 4, top row). Tokens and Types predicted expressive language scores in both HRA+ and HRA− groups (HRA+ Tokens: r = .69, p <.05; HRA+ Types: r =.68, p <.05; HRA− Tokens: r = .5, p = .098; HRA− Types: r =.63, p <.05) so the interaction was not significant. Diagnostic outcome did not moderate relationships between any input measure and receptive language scores (all β < 1.05, p > .18).
Figure 4.
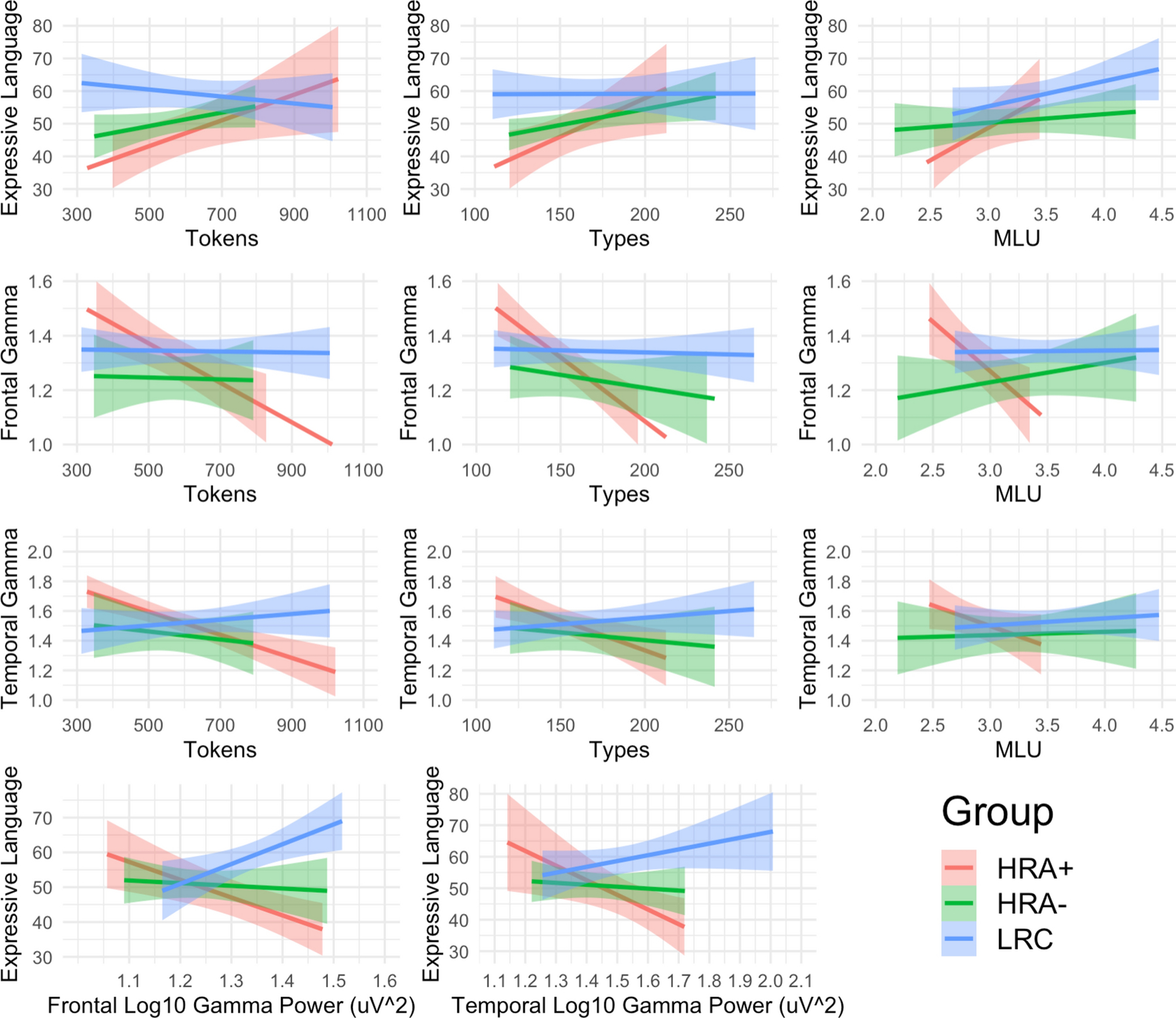
Relationships between language input, regional gamma power, and expressive language scores by risk and diagnostic group. The top row displays relationships between language input measures and expressive language scores on the Mullen Scales of Early Learning; the middle rows display relationships between language input measures and log10-transformed absolute power in the gamma band (30–50 Hz) over frontal and temporal scalp regions; and the bottom row displays the relationships between the two regional gamma power measures and expressive language scores. Shaded areas are the 95% confidence intervals. See Supplementary Figure 1 for plots with receptive language in place of expressive language. MLU= Mean Length of Utterance; LRC = low risk control; HRA+ = High risk of Autism, diagnosed with ASD; HRA− = high risk of Autism, not diagnosed with ASD.
Relationships between Input and Gamma Power
Within the whole group, no 18-month input measure significantly predicted either 24-month EEG measure. However, risk status did significantly moderate the relationships between Tokens and Types and gamma power in both frontal regions (Tokens: β = −1.05, p < .05; Types: β = −1.52, p < .01) and temporal regions (Tokens: β = −1.34, p < .05; Types: β = −1.50, p < .05). In both cases, input negatively predicted frontal gamma power (Tokens: r = −.49, p < .05, Types: r = −.59, p < .01) and temporal gamma power (Tokens: r = −.53, p < .05, Types: r = −.47, p < .05) in HRA toddlers, such that higher input was significantly related to lower gamma power, while input did not predict gamma power in LRC toddlers (Figure 3, top row).
Within the HRA group, diagnostic outcome (ASD versus no ASD) also moderated the relationships between Tokens, Types, and MLU and Frontal gamma power (Tokens: β = −2.55 p < .01; Types: β = −1.19, p < .05; MLU: β = −4.51, p < .01) and between Types and Temporal gamma power (β = −1.21, p < .05). In all cases, input negatively predicted both frontal gamma power (Tokens: r = −.82, p < .01, Types: r = −.89, p < .001, MLU: r = −.77, p < .05) and temporal gamma power (Tokens: r = −.88, p < .001, Types: r = −.77, p < .01, MLU: r = −.59, p = .07) in HRA+ toddlers, but not in HRA− toddlers (Figure 3, bottom row, and Figure 4, middle rows).
Relationships between Gamma Power and Language Scores
Within the whole group, neither 24-month regional gamma power measure correlated with cotemporaneous receptive or expressive language scores (all r < .08, all p > .6). However, risk did significantly moderate the relationships between both frontal gamma power and both receptive and expressive language scores (Receptive: β = −3.29, p < .05; Expressive: β = −5.60, p < .001), as well as between temporal gamma power and expressive language scores (β = −3.06, p < .01). In HRA toddlers, regional gamma power was negatively correlated with expressive language scores (Frontal: r = −.61, p < .01, Temporal: r = −.51, p < .05) language scores, while in LRC toddlers, frontal gamma power was positively correlated with expressive language scores (r = .51, p < .05) and temporal gamma was uncorrelated with language scores (Figure 3, top row). Neither gamma power measure was correlated with receptive language scores in either group.
Within the HRA group, diagnostic outcome (ASD versus no ASD) also moderated relationships between temporal gamma power and expressive language scores (β = −3.67, p < .01), and marginally moderated relationships between frontal gamma power and expressive language scores (β = −3.40, p = .06). In both cases, gamma power was negatively correlated with expressive language scores in HRA+ toddlers (Frontal: r = −.79, p < .01, Temporal: r = −.72, p < .05) and was uncorrelated with language scores in HRA− toddlers (Figure 3, bottom row, and Figure 4, bottom row).
Full Moderated Mediation Models
Within the full sample, no models assessing indirect or conditional indirect effects by risk status were significant because there were minimal relationships amongst independent, dependent, and mediating variables in the LRC group. Additionally, no models with an outcome measure of receptive language were significant, also because no input or neural measure was significantly associated with receptive language in any subgroup. Thus, all models reported below are conducted within HRA toddlers only, with a conditional effect of diagnostic outcome and a dependent outcome measure of expressive language scores.
First-stage moderated mediation models (model 7, Hayes, 2018) assess whether gamma power mediates the effect of input on expressive language skills and whether diagnostic outcome moderates the relationship between input and gamma power (the a path). A formal test of first-stage moderated mediation based on the index term (the difference between conditional indirect effects) revealed that HRA subgroup (HRA+ or HRA−) moderated the indirect effect of tokens on expressive language scores through frontal gamma power (B = .02, SE = .02, 95% CI = [.0002, .06]; Figure 5, top left). Specifically, the mediation was significant for HRA+ toddlers (B = .02, SE =.02, CI = [.003, .06]), but not for HRA− toddlers (B = .0009, SE = .008, CI = [−.01, .02]). No first-stage models with types were significant. The first-stage models with MLU through both frontal and temporal gamma power were significant, but the combined first and second stage models had a higher R-squared values (frontal: +.03; temporal: +.08) and moderated mediation index terms (frontal: +3.10; temporal: +5.06; see below).
Figure 5.
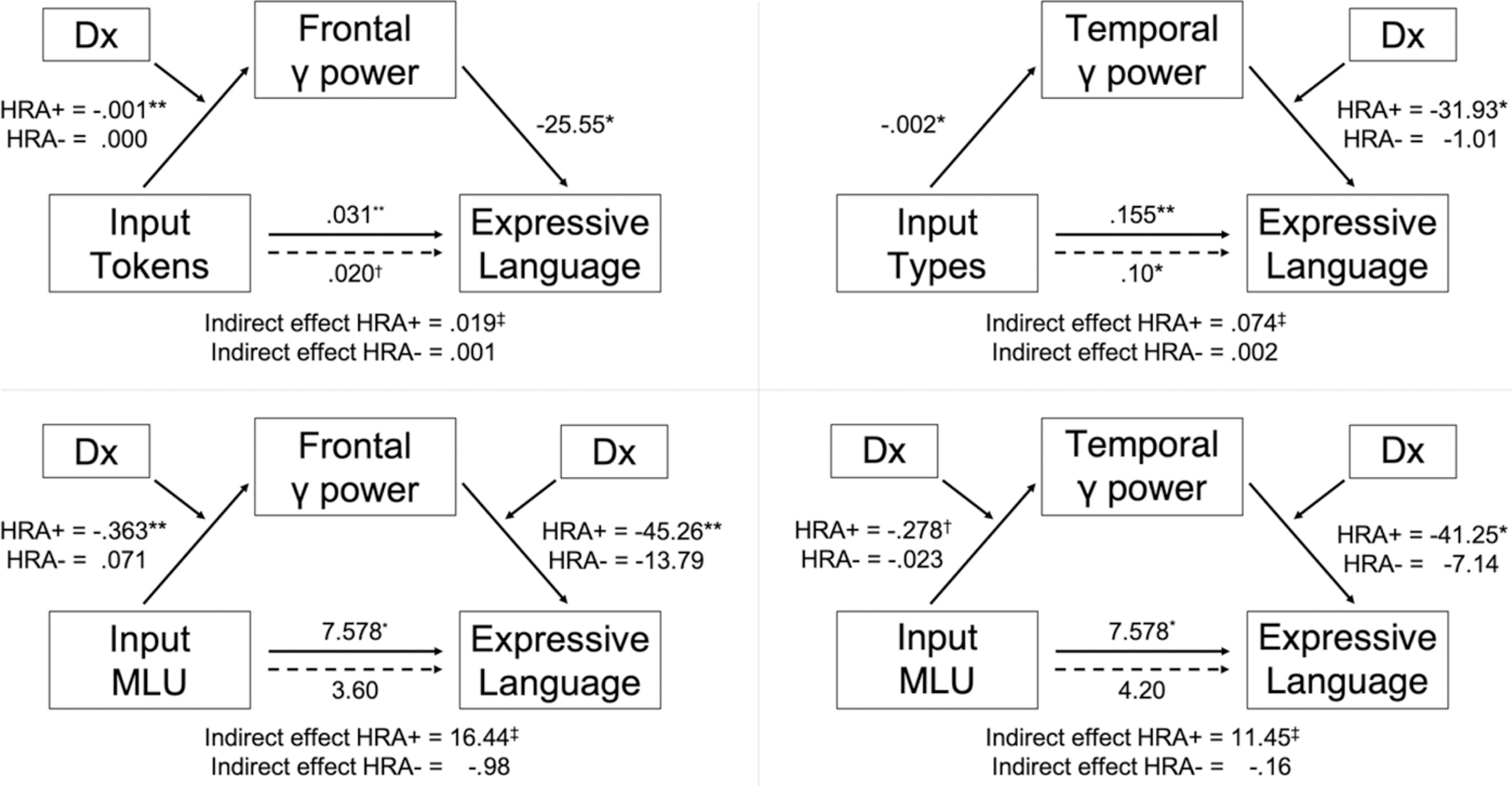
Moderated mediation models estimating whether the relationship between input and expressive language skill is mediated by regional gamma power, conditional on diagnostic outcome. Numbers on pathways indicate unstandardized regression coefficients. The index of moderated mediation is significant for all models shown (see text), and in all cases, the mediation is significant for HRA+ and not for HRA− toddlers. MLU= Mean Length of Utterance; HRA+ = High risk of Autism, diagnosed with ASD; HRA− = high risk of Autism, not diagnosed with ASD. †p < .1, *p < .05, **p < .01, ***p < .001, ‡significant by bootstrapped confidence intervals.
Second-stage moderated mediation models (model 14, Hayes, 2018) assess whether gamma power mediates the effect of input on expressive language skills and whether diagnostic outcome moderates the relationship between gamma power and expressive language scores (the b path). A formal test of second-stage moderated mediation revealed that HRA subgroup moderated the indirect effect of types on expressive language scores through temporal gamma power (B = .07, SE = .06, 95% CI = [.0001, .22]; Figure 5, top right). Specifically, the mediation was significant for HRA+ toddlers (B = .07, SE = .05, CI = [.007, .20]), but not for HRA− toddlers (B = .002, SE = .03, CI = [−.06, .05]). No other second-stage models were significant.
Combined first- and second-stage moderated mediation models (model 58, Hayes, 2018) assess whether gamma power mediates the effect of input on expressive language skills and whether diagnostic outcome moderates both the relationship between input and gamma power (the a path) and the relationship between gamma power and expressive language scores (the b path). Thus, the indirect effect is a quadratic function of the moderator. A formal test of first- and second-stage moderated mediation revealed that HRA subgroup moderated the indirect effect of MLU on expressive language scores through both frontal gamma power (B = 17.43, SE = 20.48, 95% CI = 1.41, 30.99]; Figure 5, bottom left) and through temporal gamma power (B = 11.61, SE = 8.63, CI = [1.03, 29.93]; Figure 5, bottom right). Specifically, the mediations were significant for HRA+ toddlers (frontal: B = 16.44, SE = 20.48, CI = [.40, 30.12]; temporal: B = 11.45, SE = 8.62, CI = [1.09, 29.76]), but not for HRA− toddlers (frontal: B = −.98, SE = 1.84, CI = [−4.74, 1.61]; temporal: B = −.16, SE = 1.46, CI = [−3.47, 2.05]). No other combined first- and second-stage models were significant.
Discussion
In this study we sought to determine whether the neuroscillatory mechanisms linking toddlers’ language input to their language skills vary by toddlers’ familial risk for ASD and/or by ultimate diagnostic outcomes. Results indicate that quantitative (number of words) and qualitative (lexical diversity and grammatical complexity) measures of parental language input at 18-months of age predict toddlers’ language skills 6 months later, but that these relationships are moderated by both ASD risk and diagnostic outcome. Specifically, high-risk toddlers showed stronger relationships between input and expressive language scores than low-risk toddlers, and high-risk toddlers who ultimately receive ASD diagnoses showed the strongest relationships. Furthermore, in high-risk toddlers, and especially those who would later receive diagnoses of autism, greater/higher quality input was associated with lower baseline gamma power over frontal and temporal regions 6-months later, which in turn was related to higher expressive language scores. Moderated mediation models revealed that relationships between input and expressive language scores were explained by frontal (types and MLU) and temporal (tokens and MLU) gamma power, but only for toddlers who received ASD diagnoses. Together, results suggest that high-risk toddlers appear to be both cognitively and neurally more sensitive to their early language environments, and the neurophysiological mechanisms underlying experience-dependent language development differ according ultimate diagnosis.
The finding that high-risk toddlers exhibit stronger effects of input on neuroscillatory patterns and language skills is consistent with a theory of differential susceptibility to one’s early environment, in which certain individuals or groups of individuals are neurobiologically more sensitive than others to both negative and positive experiences (Belsky & Pluess, 2009; Boyce, 2016; Ellis et al., 2011). By such an account, inherent to HRA children is some to-be-determined risk factor—which may be of prenatal origin, genetic, familial, and/or experientially derived—that predisposes them to be more susceptible to variation in their language input in terms of its effects on neurodevelopment. Although typically referenced in response to vulnerability to adverse early experiences, differential susceptibility theory posits that more susceptible children will also be more receptive to promotive early experiences (Ellis et al., 2011), such as positive parenting practices, or in the present case, rich early language environments. Indeed, toddlers in the HRA+ group who experienced higher quantity and quality of language input exhibited average-range language scores on par with many low-risk controls. This suggests the potential benefit of preemptive intervention programs to support caregivers’ language interactions with pre-symptomatic high-risk children to support optimal language development (Swanson, 2020).
Although correlations between gamma power and language input/language skills were hypothesized, the negative correlations (i.e., higher input was associated lower gamma power, which was in turn associated with higher language skills) in HRA toddlers were surprising. It is important to note that Wilkinson and colleagues (2019) have previously found negative correlations between frontal gamma power and concurrent language scores in a larger sample of participants of the same study as is reported here (i.e., all 24-month-olds with EEG and language scores, irrespective of 18-month parent child interactions), and the present study extends these negative associations to language input 6-months prior. Of the studies finding relationships between gamma power and language development in typically developing children, all have reported positive correlations, with higher gamma power predicting better language outcomes (Benasich et al., 2008; Brito et al., 2016; Cantiani et al., 2019; Gou et al., 2011; Tarullo et al., 2012; Tarullo et al., 2017; although, note that Wilkinson et al, 2019 did not find a significant association between frontal gamma and language scores in LRC children). Additionally, the emerging literature relating language input to infant brain oscillations also finds positive associations between input and high-frequency brain rhythms (Brito et al., 2020; Pierce et al., 2020). However, the present findings of opposite (i.e., negative) associations are consistent with previous studies that have found reversed brain-language relationships in toddlers at risk of and/or diagnosed with ASD. For example, in a prospective functional magnetic resonance imaging (fMRI) study of 1–3 year-olds, associations between language-related brain activation and later language development were in opposite directions in children who would and would not develop ASD (Lombardo et al., 2015). While the reason for the reversed findings in high-risk toddlers is unclear, it may originate in the already atypical neural circuitry in these children. Specifically, because ASD is hypothesized to stem from imbalances in neural excitation/inhibition (Geschwind & Levitt, 2007; Rubenstein & Merzenich, 2003), a reduction in gamma power may indicate successful compensation to hypoactive inhibitory neurons (Wilkinson et al., 2019). The present study suggests that greater quantity and quality of language input may support these neurobiological processes to support language development in high-risk children.
Additionally, there were slight differences amongst the relationships between measures of language input and gamma power in the HRA+ toddlers. Specifically, input tokens (a measure of the sheer quantity of input) were more strongly related to gamma power in the temporal region, while input types (a measure of lexical diversity) and MLU (a measure of grammatical complexity) were more strongly related to gamma power in the frontal region. This pattern may reflect different relationships between input quantity/quality and different language regions in the brain. The temporal cortex houses early developing regions responsible for auditory processing and lower-level receptive language processing, while the prefrontal cortex subserves later-developing semantic and syntactic processing, speech production, and other higher-level cognitive processes. Although speculative, it is possible that sheer input quantity has the largest effects on lower-level language-related brain development in temporal regions, while input quality has greater effects on higher-level language-related development in prefrontal regions. This would be consistent with developmental trajectories of the variable influence of language input, in which quantitative measures are most important for young infants, while qualitative measures become increasingly more important as children age (Rowe, 2012; Rowe & Snow, 2020). Further, this would suggest that early interventions to support caregivers’ language input to high-risk children should be tailored to the child’s age and developmental level, and should focus not only on responsiveness, but also the quantity, diversity, and complexity of input.
The present findings also raise questions about why caregivers vary in their language input to children. Even though the language input measures temporally preceded the biobehavioral measures, there are likely reciprocal relationships between parent input and child development. Specifically, as high-risk children age and exhibit increasingly more phenotypic ASD characteristics, parents may tailor their input to match their child’s developmental language level (Bottema-Beutel & Kim, 2020; Leezenbaum et al., 2014). The present study focused only on parent-to-child input effects in service of identifying neurobiological links, yet further research is necessary to characterize the nature of reciprocal relationships between caregiver speech and child language development in higher-risk children, and the impact of caregiver support interventions across a variety of caregivers with differing communication styles.
Despite its strengths, this study has several limitations. First, the language input measure was derived from short, in-lab video recordings which may not be fully representative of a child’s full day-to-day experience (Bergelson et al., 2019). Many language development studies have begun utilizing daylong audio recordings to provide automated measures of children’s naturalistic language experience (Ganek & Eriks-Brophy, 2018). However, at present these measures only provide quantitative information on children’s language input (e.g., the number of words and conversational turns), while human transcription is still considered the gold standard for deriving in-depth qualitative input measures, which appear to have unique relations to language development in the present study and others (Rowe & Snow, 2020). Second, we explored multiple measures for each construct of interest (three input, two EEG, and two language) because we did not have strong hypotheses about which would be most related to the other variables and did not want to overlook non-hypothesized relationships. Because this was exploratory, we did not correct for multiple comparisons, so results should be replicated with the implicated measures only for greater confidence. Additionally, the overall sample size was not large, which made the subgroups, and especially the two HRA subgroups, even smaller. Although the effect sizes within the HRA+ subgroup were strong (correlations between input, gamma power, and expressive language ranged from .65 to .89), results should be replicated with a larger sample. Finally, the sample skewed toward non-Hispanic white, highly educated, and higher income families, which may limit generalization to more diverse populations of children. This also may have constrained the observed range in input measures, which could have contributed to the limited correlations between input and language scores in the LRC group (though see Choi et al., 2020 for relationships within the full sample of transcribed interactions, irrespective of usable EEG data). While such sample demographics are unfortunately not unusual in developmental neuroscience research, further research with more representative samples is critical to a comprehensive understanding of the neural mechanisms underlying language development in autism.
In conclusion, this study revealed that toddlers at high risk of ASD, and especially those who go on to receive ASD diagnoses, are disproportionately sensitive to the quantity and quality of their early language environments, and that the neuroscillatory mechanisms mediating these input-language associations differ between toddlers who do and do not develop ASD. Beyond the mechanistic value, these findings also have strong translational implications. Specifically, they highlight the importance of early (i.e., pre-symptomatic) intervention programs to support caregivers in providing high-quality language to high-risk children to optimally scaffold their language development.
Supplementary Material
Supplementary Figure 1. EEG acquisition nets and electrodes used in analyses. The 128-channel EGI HydroCel Geodesic Sensor Net (version 1.0), top panel, and 64- channel EGI Geodesic Sensor Net (version 2.0), bottom panel, used in the study. Electrodes included in the frontal region of interest (ROI) are shown in red, and those in the temporal ROI are shown in blue.
Supplementary Figure 2. Relationships between language input, regional gamma power, and receptive language scores by risk and diagnostic group. The top row displays relationships between language input measures and receptive language scores on the Mullen Scales of Early Learning; the middle rows display relationships between language input measures and log10-transformed absolute power in the gamma band (30–50 Hz) over frontal and temporal scalp regions; and the bottom row displays the relationships between the two regional gamma power measures and receptive language scores. Shaded areas are the 95% confidence intervals See Figure 3 (main text) for plots with expressive language in place of receptive language. MLU= Mean Length of Utterance; LRC = low risk control; HRA+ = High risk of Autism, diagnosed with ASD; HRA− = high risk of Autism, not diagnosed with ASD.
Acknowledgments:
We would like to thank all children and families who participated in this study as well as the former and current Infant Sibling Project team members for their help in the data collection. We would also like to thank Priyanka Shah, Phoebe Stoye, and Aine Scholand for assistance in transcribing parent-child interaction videos.
Funding:
This study was funded by the grants from the National Institutes of Health (R01-DC010290 to HTF and CAN; R21-DC08637 to HTF; and T32MH112510 to RRR and CLW), the Autism Science Foundation (to LG-D, CLW, and AL), the Rett Syndrome Research Foundation (to LG-D), the University of Tokyo International Research Center for Neurointelligence (to LG-D), the American Brain Foundation (to AL), the Nancy Lurie Marks Family Foundation (to AL), the Brain and Behavior Research Foundation (to AL), Autism Speaks (1323 to HTF), and Simons Foundation (137186 to CAN). The funding bodies did not have any role in the design, collection, analyses, and interpretation of data or in writing the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors declare that they have no conflict of interests.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Data Availability:
Data for this study is available from the NIMH Data Archive at https://nda.nih.gov/edit_collection.html?id=1900 Materials and Code are available upon request to the first author.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Anderson AJ, & Perone S (2018). Developmental change in the resting state electroencephalogram: Insights into cognition and the brain. Brain Cogn, 126, 40–52. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, … Pickles A (2007). Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol, 75(4), 594–604. [DOI] [PubMed] [Google Scholar]
- Babadi B, & Brown EN (2014). A review of multitaper spectral analysis. IEEE Trans Biomed Eng, 61(5), 1555–1564. [DOI] [PubMed] [Google Scholar]
- Bang J, & Nadig A (2015). Learning language in autism: Maternal linguistic input contributes to later vocabulary. Autism Research, 8(2), 214–223. [DOI] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull, 135(6), 885–908. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, & Harris KD (2008). Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav Brain Res, 195(2), 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Burraco A, & Murphy E (2019). Why brain oscillations are improving our understanding of language. Front Behav Neurosci, 13, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson E, Amatuni A, Dailey S, Koorathota S, & Tor S (2019). Day by day, hour by hour: Naturalistic language input to infants. Dev Sci, 22(1), e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, & Gillberg C (2005). Autism after adolescence: Population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord, 35(3), 351–360. [DOI] [PubMed] [Google Scholar]
- Bottema-Beutel K, & Kim SY (2021). A systematic literature review of autism research on caregiver talk. Autism Res, 14(3):432–449. [DOI] [PubMed] [Google Scholar]
- Boyce WT (2016). Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology, 41(1), 142–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, eeg power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Troller-Renfree SV, Leon-Santos A, Isler JR, Fifer WP, & Noble KG (2020). Associations among the home language environment and neural activity during infancy. Dev Cogn Neurosci, 43, 100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C, Piazza C, Mornati G, Molteni M, & Riva V (2019). Oscillatory gamma activity mediates the pathway from socioeconomic status to language acquisition in infancy. Infant Behav Dev, 57, 101384. [DOI] [PubMed] [Google Scholar]
- Choi B, Nelson CA, Rowe ML, & Tager-Flusberg H (2020). Reciprocal influences between parent input and child language skills in dyads involving high- and low-risk infants for autism spectrum disorder. Autism Res, 13(7), 1168–1183. [DOI] [PubMed] [Google Scholar]
- Csibra G, Davis G, Spratling MW, & Johnson MH (2000). Gamma oscillations and object processing in the infant brain. Science, 290(5496), 1582–1585. [DOI] [PubMed] [Google Scholar]
- deBoer T, Scott LS, & Nelson CA (2007). Methods for acquiring and analysing infant event-related potentials. In de Haan M (Ed.), Infant eeg and event-related potentials. East Sussex, UK: Psychology Press. [Google Scholar]
- Edwards JR, & Lambert LS (2007). Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychol Methods, 12(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, & van Ijzendoorn MH (2011). Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Dev Psychopathol, 23(1), 7–28. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2005). Neurophysiological markers of early language acquisition: From syllables to sentences. Trends Cogn Sci, 9(10), 481–488. [DOI] [PubMed] [Google Scholar]
- Fries P (2009). Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual Review of Neuroscience, 32(1), 209–224. [DOI] [PubMed] [Google Scholar]
- Fusaroli R, Weed E, Fein D, & Naigles L (2019). Hearing me hearing you: Reciprocal effects between child and parent language in autism and typical development. Cognition, 183, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, & Levin AR (2018). The harvard automated processing pipeline for electroencephalography (happe): Standardized processing software for developmental and high-artifact data. Frontiers in Neuroscience, 12(97). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Wilkinson C, Kapur K, Tager-Flusberg H, Levin AR, & Nelson CA (2019). Longitudinal eeg power in the first postnatal year differentiates autism outcomes. Nature Communications, 10(1), 4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganek H, & Eriks-Brophy A (2018). Language environment analysis (lena) system investigation of day long recordings in children: A literature review. Journal of Communication Disorders, 72, 77–85. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, & Levitt P (2007). Autism spectrum disorders: Developmental disconnection syndromes. Curr Opin Neurobiol, 17(1), 103–111. [DOI] [PubMed] [Google Scholar]
- Gilkerson J, Richards JA, Warren SF, Oller DK, Russo R, & Vohr B (2018). Language experience in the second year of life and language outcomes in late childhood. Pediatrics, 142(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2012). Trajectories of autism severity in children using standardized ados scores. Pediatrics, 130(5), e1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, & Benasich AA (2011). Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav Brain Res, 220(2), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50, 1–22. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis: A regression-based perspective (2nd ed.). New York, NY: The Guilford Press. [Google Scholar]
- Howlin P, Goode S, Hutton JS, & Rutter M (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, 45(2), 212–229. [DOI] [PubMed] [Google Scholar]
- Jochaut D, Lehongre K, Saitovitch A, Devauchelle A-D, Olasagasti I, Chabane N, … Giraud A-L (2015). Atypical coordination of cortical oscillations in response to speech in autism. Frontiers in Human Neuroscience, 9(171). [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Camacho MC, Montez DF, Humphreys KL, & Gotlib IH (2021). Naturalistic language input is associated with resting-state functional connectivity in infancy. J Neurosci, 41(3), 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnik A, Begum Ali J, Gliga T, Guiraud J, Charman T, Johnson MH, … The BT (2019). Increased cortical reactivity to repeated tones at 8 months in infants with later asd. Translational Psychiatry, 9(1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leezenbaum NB, Campbell SB, Butler D, & Iverson JM (2014). Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism, 18(6), 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Méndez Leal AS, Gabard-Durnam LJ, & O’Leary HM (2018). Beapp: The batch electroencephalography automated processing platform. Frontiers in Neuroscience, 12, 513–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, & Nelson CA (2015). Inhibition-based biomarkers for autism spectrum disorder. Neurotherapeutics, 12(3), 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Varcin KJ, O’Leary HM, Tager-Flusberg H, & Nelson CA (2017). EEG power at 3 months in infants at high familial risk for autism. J Neurodev Disord, 9(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C, Solso S, … Courchesne E (2015). Different functional neural substrates for good and poor language outcome in autism. Neuron, 86(2), 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- MacWhinney B (2000). The childes project: Tools for analyzing talk. (3rd ed. Vol. 2: The Database). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Marrus N, Hall LP, Paterson SJ, Elison JT, Wolff JJ, Swanson MR, … Ibis Network. (2018). Language delay aggregates in toddler siblings of children with autism spectrum disorder. J Neurodev Disord, 10(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Maskus EA, Melvin SA, He X, & Noble KG (2020). Socioeconomic disparities in language input are associated with children’s language-related brain structure and reading skills. Child Dev, 91, 846–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, … Zwaigenbaum L (2015). Early sex differences are not autism-specific: A baby siblings research consortium (bsrc) study. Mol Autism, 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Burke JD, Troyb E, Knoch K, Herlihy LE, & Fein DA (2017). Preschool predictors of school-age academic achievement in autism spectrum disorder. Clin Neuropsychol, 31(2), 382–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Naigles LR (2013). Input and language development in children with autism. Seminars in speech and language, 34(4), 237–248. [DOI] [PubMed] [Google Scholar]
- Nelson CA, & McCleery JP (2008). Use of event-related potentials in the study of typical and atypical development. J Am Acad Child Adolesc Psychiatry, 47(11), 1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, & Elam M (2007). Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry, 62(9), 1022–1029. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Cantiani C, Shafer VL, & Benasich AA (2019). Minimally-verbal children with autism show deficits in theta and gamma oscillations during processing of semantically-related visual information. Scientific Reports, 9(1), 5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Hämäläinen JA, Realpe-Bonilla T, & Benasich AA (2016). Oscillatory dynamics underlying perceptual narrowing of native phoneme mapping from 6 to 12 months of age. J Neurosci, 36(48), 12095–12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL (2011). Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics, 128(3), e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif AM (2015). Diagnostic stability in young children at risk for autism spectrum disorder: A baby siblings research consortium study. J Child Psychol Psychiatry, 56(9), 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce LJ, Reilly E, & Nelson CA (2020). Associations between maternal stress, early language behaviors, and infant electroencephalography during the first year of life. J Child Lang, 1–28. [DOI] [PubMed]
- Pivik RT, Andres A, Cleves MA, Tennal KB, Gu Y, & Badger TM (2018). Developmental changes in resting gamma power from age three months to five years are modulated by infant diet. The FASEB Journal, 31(S1), 958.959–958.959. [Google Scholar]
- Pivik RT, Andres A, Tennal KB, Gu Y, Downs H, Bellando BJ, … Badger TM (2019). Resting gamma power during the postnatal critical period for gabaergic system development is modulated by infant diet and sex. International Journal of Psychophysiology, 135, 73–94. [DOI] [PubMed] [Google Scholar]
- Romeo RR, Leonard JA, Grotzinger H, Robinson ST, Takada M, Mackey AP, … Gabrieli JDE (2020). Neuroplasticity associated with conversational turn-taking following a family-based intervention. [DOI] [PMC free article] [PubMed]
- Romeo RR, Leonard JA, Robinson ST, West MR, Mackey AP, Rowe ML, & Gabrieli JDE (2018). Beyond the “30 million word gap:” children’s conversational exposure is associated with language-related brain function. Psychological Science, 29(5), 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RR, Segaran J, Leonard JA, Robinson ST, West MR, Mackey AP, … Gabrieli JDE (2018). Language exposure relates to structural neural connectivity in childhood. Journal of Neuroscience, 38(36), 7870–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML (2012). A longitudinal investigation of the role of quantity and quality of childdirected speech in vocabulary development. Child Dev, 83(5), 1762–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML, & Snow CE (2020). Analyzing input quality along three dimensions: Interactive, linguistic, and conceptual. Journal of Child Language, 47(1), 5–21. [DOI] [PubMed] [Google Scholar]
- Rowe ML, & Weisleder A (2020). Language development in context. Annual Review of Developmental Psychology, 2(1), 201–223. [Google Scholar]
- Rubenstein JL, & Merzenich MM (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav, 2(5), 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The social communication questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Saby JN, & Marshall PJ (2012). The utility of eeg band power analysis in the study of infancy and early childhood. Dev Neuropsychol, 37(3), 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller M, & Sigman M (2002). The behaviors of parents of children with autism predict the subsequent development of their children’s communication. Journal of Autism and Developmental Disorders, 32(2), 77–89. [DOI] [PubMed] [Google Scholar]
- Skeide MA, & Friederici AD (2016). The ontogeny of the cortical language network. Nat Rev Neurosci, 17(5), 323–332. [DOI] [PubMed] [Google Scholar]
- Swanson MR (2020). The role of caregiver speech in supporting language development in infants and toddlers with autism spectrum disorder. Dev Psychopathol, 32(4), 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MR, Donovan K, Paterson S, Wolff JJ, Parish-Morris J, Meera SS, … Network, I. (2019). Early language exposure supports later language skills in infants with and without autism. Autism Res, 12(12), 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen LD, Naigles LR, & Fein D (2007). Does maternal input affect the language of children with autism? Paper presented at the Proceedings of the 31st Annual bsoton University Conference on Language Development, Somerville, MA. [Google Scholar]
- Tager-Flusberg H (2006). Defining language phenotypes in autism. Clinical Neuroscience Research, 6(3–4), 219–224. [Google Scholar]
- Tager-Flusberg H (2016). Risk factors associated with language in autism spectrum disorder: Clues to underlying mechanisms. J Speech Lang Hear Res, 59(1), 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Edelson L, & Luyster R (2011). Language and communication in autism spectrum disorders. In Amaral D, Dawson G, & Geschwi D.nd (Eds.), Autism spectrum disorders (pp. (pp. 172–185).). Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Tager-Flusberg H, & Kasari C (2013). Minimally verbal school-aged children with autism spectrum disorder: The neglected end of the spectrum. Autism Research, 6(6), 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, & Lord C (2005). Language and communication in autism. In Volkmar F, Paul R, Klin A, & Cohen D (Eds.), Handbook of autism and pervasive developmental disorders (pp. 335–364). Indianapolis, IN: Wiley. [Google Scholar]
- Takano T, & Ogawa T (1998). Characterization of developmental changes in eeg-gamma band activity during childhood using the autoregressive model. Acta Paediatr Jpn, 40(5), 446–452. [DOI] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, & Tager-Flusberg H (2016). Maternal vocal feedback to 9-month-old infant siblings of children with asd. Autism Res, 9(4), 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Lee J, Gera S, Condon C, Tamura EK, Grieve PG, … Fifer WP (2012). Language development linked to newborn frontal gamma power. Paper presented at the International Society for Developmental Psychobiology. [Google Scholar]
- Tarullo AR, Obradovic J, Keehn B, Rasheed MA, Siyal S, Nelson CA, & Yousafzai AK (2017). Gamma power in rural pakistani children: Links to executive function and verbal ability. Dev Cogn Neurosci, 26, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney A, Strait DL, O’Connell S, & Kraus N (2013). Developmental changes in resting gamma power from age three to adulthood. Clin Neurophysiol, 124(5), 1040–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2012). Developmental trajectories of resting eeg power: An endophenotype of autism spectrum disorder. PLoS One, 7(6), e39127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, … Kushnerenko E (2013). Socioeconomic status and functional brain development - associations in early infancy. Dev Sci, 16(5), 676–687. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, & Singer W (2010). Neural synchrony and the development of cortical networks. Trends Cogn Sci, 14(2), 72–80. [DOI] [PubMed] [Google Scholar]
- Warren SF, Gilkerson J, Richards JA, Oller DK, Xu D, Yapanel U, & Gray S (2010). What automated vocal analysis reveals about the vocal production and language learning environment of young children with autism. J Autism Dev Disord, 40(5), 555–569. [DOI] [PubMed] [Google Scholar]
- Wilkinson CL, Gabard-Durnam LJ, Kapur K, Tager-Flusberg H, Levin AR, & Nelson CA (2020). Use of longitudinal eeg measures in estimating language development in infants with and without familial risk for autism spectrum disorder. Neurobiol Lang (Camb), 1(1), 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CL, Levin AR, Gabard-Durnam LJ, Tager-Flusberg H, & Nelson CA (2019). Reduced frontal gamma power at 24 months is associated with better expressive language in toddlers at risk for autism. Autism Res, 12(8), 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Brandl S, Horn F, Waldburger E, Allefeld C, & Tangermann M (2014). Robust artifactual independent component classification for bci practitioners. J Neural Eng, 11(3), 035013. [DOI] [PubMed] [Google Scholar]
- Xie W, & Nelson CA (2021). The state-of-the-art methodological review of pediatric eeg. In Huang H & Robert T (Eds.), Handbook of paediatric brain imaging: Methods, modalities and applications. London, UK: Elsevier Press. [Google Scholar]
- Zwaigenbaum L, Bryson SE, Brian J, Smith IM, Roberts W, Szatmari P, … Vaillancourt T (2016). Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res, 9(7), 790–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. EEG acquisition nets and electrodes used in analyses. The 128-channel EGI HydroCel Geodesic Sensor Net (version 1.0), top panel, and 64- channel EGI Geodesic Sensor Net (version 2.0), bottom panel, used in the study. Electrodes included in the frontal region of interest (ROI) are shown in red, and those in the temporal ROI are shown in blue.
Supplementary Figure 2. Relationships between language input, regional gamma power, and receptive language scores by risk and diagnostic group. The top row displays relationships between language input measures and receptive language scores on the Mullen Scales of Early Learning; the middle rows display relationships between language input measures and log10-transformed absolute power in the gamma band (30–50 Hz) over frontal and temporal scalp regions; and the bottom row displays the relationships between the two regional gamma power measures and receptive language scores. Shaded areas are the 95% confidence intervals See Figure 3 (main text) for plots with expressive language in place of receptive language. MLU= Mean Length of Utterance; LRC = low risk control; HRA+ = High risk of Autism, diagnosed with ASD; HRA− = high risk of Autism, not diagnosed with ASD.
Data Availability Statement
Data for this study is available from the NIMH Data Archive at https://nda.nih.gov/edit_collection.html?id=1900 Materials and Code are available upon request to the first author.


