Figure 4.
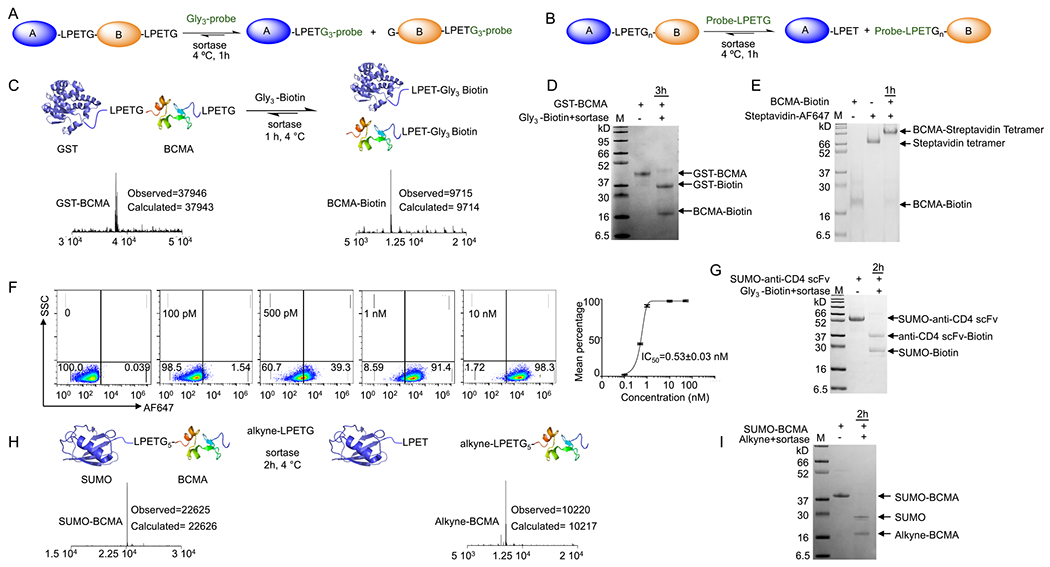
Simultaneous cleavage and site-specific C- or N-terminal protein labeling using sortase. (A,B) Schematic representation of simultaneous cleavage and site-specific C- or N-terminal protein labeling using sortase. (C) GST-BCMA fusion protein was engineered to contain a sortase recognition sequence between the two proteins and at the C terminus; addition of sortase and Gly3-biotin substrate resulted in simultaneous and near-quantitative cleavage and formation of C-terminally biotin-labeled BCMA; LC-MS analyses confirmed the formation of the products. (D) SDS-PAGE confirmed the formation of the products. (E,F) BCMA-biotin was tetramerized using streptavidin. Flow cytometry analyses on BCMA-CAR Jurkat T cells confirmed retention of BCMA functionality (EC50 = 0.53 nM). (G) SUMO-anti-CD4 scFv fusion protein was engineered to contain a sortase recognition sequence between the two proteins and at the C terminus; addition of sortase and Gly3-biotin substrate resulted in simultaneous and near-quantitative cleavage and formation of the C-terminally biotin-labeled anti-CD4 scFv; SDS-PAGE confirmed the formation of the products. (H) SUMO-BCMA fusion with an LPETG4 sequence between the SUMO and BCMA was produced by periplasmic expression in E. coli. Addition of alkyne-functionalized LPETG substrate and sortase yielded near-quantitative cleavage and facilitated N-terminally alkyne-labeled BCMA. (I) SDS-PAGE confirmed the formation of the products.
