Abstract
Background and aims
Recent studies have reported poor humoral immune response to mRNA vaccines in patients with chronic liver disease (CLD). However, the immunogenicity of ChAdOx1 (vector-based) and BBV152 (inactivated virus) vaccines in patients with CLD and liver transplant recipients (LTRs) is unknown. Therefore, we aimed to assess the immunogenicity of ChAdOx1 and BBV152 vaccines in patients with CLD (including cirrhosis patients) and LTRs.
Methods
In this single-center prospective study, consecutive completely vaccinated (ChAdOx1 or BBV152) non-cirrhosis CLD patients, those with cirrhosis, and LTRs were compared with matched healthy controls for anti-spike antibody and cellular response.
Results
Sixty healthy individuals, 50 NCCLD patients, 63 compensated and 50 decompensated cirrhosis, and 17 LTRs were included. The proportion of non-responders was similar among the healthy control (8 %), non-cirrhosis CLD (16 %), and compensated cirrhosis groups (17.5 %;p = 0.3). However, a higher proportion of patients with decompensated cirrhosis (34 %) and LTRs (59 %) were non-responders than the healthy controls (p = 0.001). Cluster of differentiation (CD) 4-effector cells were lower in patients with non-cirrhosis CLD and compensated cirrhosis. CD4-naïve, CD4-effector, B, and B-memory cells were lower in the decompensated cirrhosis group. Although the central memory cells were higher in the decompensated cirrhosis group, they could not differentiate into effector cells. CD4- and CD8-naïve cells were higher in the marrow in the LTRs, while the CD4-effector memory cells and CD4- and CD8-effector cells were lower in the LTRs. Furthermore, B cells were more deficient in the LTRs, suggesting poor antibody response.
Conclusion
Patients with decompensated cirrhosis and LTRs demonstrated suboptimal humoral and cellular immune responses against recombinant and inactivated COVID-19 vaccines.
Keywords: COVID-19 vaccines, Antibody formation, Liver cirrhosis, Humoral immunity, Cellular immunity
Abbreviations: AU, arbitrary units; CD, cluster of differentiation; CLD, chronic liver disease; COVID-19, coronavirus disease; HVOTO, hepatic venous outflow tract obstruction; LDLT, living donor liver transplantation; LTR, liver transplant recipients; MELD NA, model for end-stage liver disease sodium; mTOR, mechanistic target of rapamycin; NAFLD/NASH, non-alcoholic fatty liver disease/non-alcoholic steatohepatitis; NCCLD, non-cirrhosis chronic liver disease; RT-PCR, reverse transcriptase-polymerase chain reaction test; SARS CoV-2, severe acute respiratory syndrome coronavirus 2
1. Introduction
Patients with chronic liver disease (CLD), including with and without cirrhosis and liver transplant recipients (LTRs), constitute a unique population and can have poorer outcomes if infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). [1], [2], [3], [4], [5] Coronavirus disease (COVID-19) vaccination can prevent infection, reduce disease severity, and improve outcomes in those with CLD and LTRs. [5], [6], [7], [8] Global hepatology and transplant societies recommend COVID-19 vaccination for all patients with CLD and LTRs. [9], [10] Previous studies have reported lower antibody response to spike protein in patients with CLD and LTR. [11], [12] Approximately 61 % of LTRs and 24 % of CLD patients had suboptimal-to-poor antibody responses to mRNA vaccines. [11] Furthermore, LTRs on anti-metabolites therapy have a lower immune response than those not on the therapy. [13] To our knowledge, no studies have assessed both humoral and cellular immune responses to recombinant (ChAdOx1 nCoV-19; Covishield) and inactivated vaccines (BBV152; Covaxin) among patients with CLD and LTRs, and compared with healthy individuals. We, therefore, aimed to compare the humoral and cellular immune responses of patients with non-cirrhosis CLD, patients with cirrhosis, and LTRs with healthy controls.
1.1. Objectives
The primary objective of the study was to assess the anti-spike antibody and cellular immune response to COVID-19 vaccines among patients with CLD, including cirrhosis patients, and then also compare the responses with healthy controls. The secondary objective was to compare the anti-spike antibody and cellular immune response among LTRs with healthy controls and, to assess the number of patients developing breakthrough infections post-vaccination.
2. Methods
2.1. Study design and center
This single-center prospective study was conducted at AIG Hospitals, Hyderabad, India, from 12th June 2021 to 7th November 2021. The study was approved by the institutional ethics committee vide letter number AIG/IEC-BH&R 16(a)/07.2021-05. The study protocol adhered to the modified ethical guidelines of the Declaration of Helsinki (1975) and written informed consent was obtained from each individual recruited for the study.
2.2. Study population
Consecutive, completely vaccinated patients with CLD, including cirrhosis patients and LTRs attending the outpatient department of AIG Hospitals, were included. In addition, healthy attendants of the patients without liver disease, healthy individuals attending the outpatient department for non-specific symptoms, and health care individuals without liver disease were included as healthy controls. We included patients and healthy individuals aged between 18 and 75 years. We excluded those who did not complete the vaccination, those with renal failure (serum creatinine > 2 mg/dL), those with symptoms of active COVID-19 infection or recent exposure to a known positive patient with a positive reverse transcriptase-polymerase chain reaction test (RT-PCR), individuals with less than two weeks from the last vaccination dose, individuals with a history of COVID-19 infection in the previous six months and those not willing for the study.
The study included four groups which were-.
group 1: healthy controls,
group 2: non-cirrhosis CLD patients,
group 3: patients with cirrhosis (compensated and decompensated), and.
group 4: LTRs.
2.3. Follow up
The baseline demographic data, including age, sex, etiology of liver disease, severity score (Child-Pugh score, model for end-stage liver disease sodium [MELD NA]), comorbidities, and drug history, were noted along with baseline biochemical parameters including complete blood count, liver function test, international normalized ratio, and kidney function test. Three milliliters of blood was collected in plain and ethylenediaminetetraacetic acid (EDTA) tubes to analyze the antibody and cellular responses. The patients were followed up for three months (from the day of last vaccination) to record if any vaccinated individuals developed COVID-19 infection. The infection severity was assessed per the current Indian council for medical research (ICMR) recommendations.
2.4. Funding
The study was investigator-initiated and was funded by the AIG Healthcare Foundation.
2.5. Humoral response
The humoral response was assessed by the LIAISON SARS-CoV-2 S1/S2 IgG. LIAISON® XL (DiaSorin, Saluggia, Italy) is a chemiluminescence immunoassay (CLIA) method for quantitatively detecting IgG anti-S1 and IgG anti-S2 antibodies to SARS-CoV-2. LIAISON® XL has intra-, and interassay precision ranges between 0 % and 4 %, with a sensitivity and specificity of 97.4 % and 98.5 %, respectively. The LIASON ELISA method has 94.4 % positive agreement with plaque reduction neutralization tests (PRNT) which is the gold standard. The antibody concentration is expressed as arbitrary units (AU/mL). Concentrations of <15.0 AU/mL are interpreted as negative, and ≥15.0 AU/mL as positive.
2.6. Cellular immune response
2.6.1. Isolation of peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated from EDTA whole blood (3 mL) by density-gradient centrifugation (Ficoll 1.077 g/mL; HiSep, Himedia). Whole blood is diluted in a 1:1 ratio with phosphate-buffered saline (PBS) and overlayed gently on Ficoll. [14] After centrifugation at 2000 rpm for 30 min, the middle layer of the buffy coat was separated and washed with 2 % fetal bovine serum (FBS) and PBS, and the final cell pellet was suspended in the same buffer for flow cytometry staining and analysis.
3. T- and B-lymphocyte phenotype characterization of PBMCs
PBMCs were characterized phenotypically by flow cytometry for T- and B-lymphocytes along with memory phenotype with cluster of differentiation (CD) 3 (FITC), CD4 (Percp Cy 5.5), CD8 (APC-Cy7), CD45RA (PE-CF594), and CCR7 (PE) for T-lymphocyte characterization and CD45 (APC-Cy7), CD20(PE), and CD27 (APC-R700) for B-lymphocyte characterization. PBMCs were stained with T- and B-lymphocyte antibody staining mix and incubated at room temperature for 30 min. After Incubation, the cells were washed with a staining buffer and acquired for flow data. Cell population analysis was performed using direct immunofluorescence with a FACS Aria Fusion flow cytometer (BD), and the data were analyzed using BD FACS Diva software.
3.1. Definitions
Completely vaccinated were defined as the recipients of two doses of either of the available vaccines (ChAdOx1 nCoV-19 (Covishield) and BBV152 (Covaxin).
Non-cirrhosis CLD patients were defined as those with previously diagnosed CLD based on clinical history, imaging, or histology. The absence of cirrhosis was documented by transient elastography.
Patients with cirrhosis were defined as those with previously diagnosed cirrhosis based on the clinical history of decompensation, imaging, or histology. CLD/cirrhosis due to any cause, including chronic hepatitis B, hepatitis C, alcohol-related liver disease (ARLD), and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) were included. Autoimmune hepatitis, Wilson's disease, hepatic venous outflow tract obstruction (HVOTO), and cryptogenic causes of CLD were grouped as others.
The presence of jaundice (bilirubin > 3 mg/dL), ascites, hepatic encephalopathy, and/or acute variceal bleeding was considered decompensation. The absence of these was considered compensated cirrhosis patients.
COVID-19 infection was defined as the presence of fever, cold, cough, and undue fatigue with positive SARS-CoV-2 RT-PCR with/without a history of exposure to a SARS-CoV-2-positive patient.
LTR was defined as a patient who has undergone liver transplantation for any etiology/indication.
Healthy controls were defined as individuals without any liver disease, irrespective of the presence or absence of comorbidities, including diabetes mellitus, hypertension, hypothyroidism, and coronary artery disease. Absence of liver disease was confirmed based on clinical history, ultrasonography, and/or transient elastography.
Obesity was defined as body mass index > 25 kg/m2.
Individuals with anti-spike antibody concentrations of < 15.0 AU/mL are considered non-responders to the vaccine.
Detection of SARS CoV-2 on RT-PCR on or after eleven days of complete vaccination in the absence of any explicit symptoms suggestive of pre-existing COVID-19 within six days of vaccination was considered a breakthrough infection. [15].
3.2. Statistical analysis
Descriptive statistics are expressed as mean with corresponding standard deviation (SD) and median with range for parametric and non-parametric continuous data, respectively, and number (%) for categorical data. Mean values were compared using students' t-test for two groups and analysis of variance (ANOVA) for > 2 groups; post-hoc comparison was reported using Bonferroni method. Categorical values were compared using chi-squared test or two-sided Fischer's exact test when any of the expected values in the contingency table was < 5. All statistical tests with p < 0.05 were considered significant. The data was initially entered into Microsoft Excel Ver.2205 (Redmond, Washington, USA), and the statistical tests were later performed using SPSS ver.25.0 (IBM Corp ltd, Armonk, NY).
4. Results
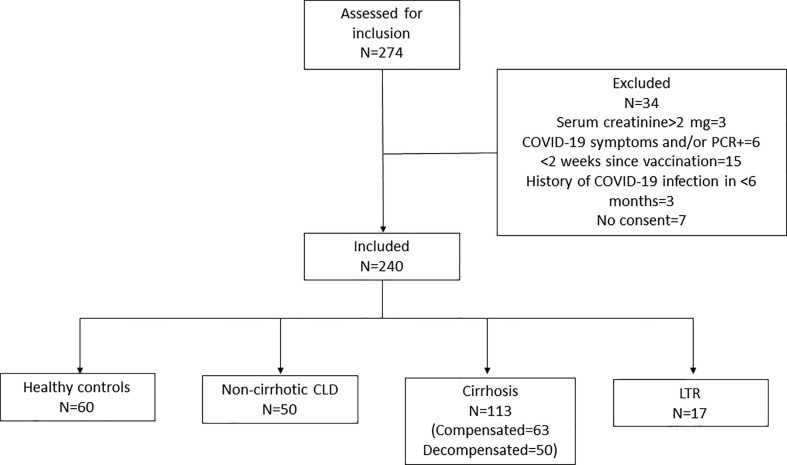
Two hundred and seventy-four patients were assessed for inclusion. Thirty-four individuals were excluded: three had serum creatinine > 2 mg/dL (one healthy and two cirrhosis); two healthy individuals had a history of exposure to a positive patient with symptoms suggestive of COVID-19; four patients with CLD tested positive (tested prior to planned endoscopic procedure) on SARS CoV-2 PCR; 15 individuals (six healthy and nine CLD patients) had recently undergone the second dose of vaccination; three patients had a history of COVID-19 infection in the previous six months, and seven individuals (four healthy and three CLD patients) were unwilling to participate in the study (Fig. 1 : Consort chart). A total of 240 individuals satisfying the inclusion criteria were included. Sixty were healthy controls; 50 were non-cirrhosis CLD patients; 113 were known cirrhosis patients, and 17 were LTRs. Age and sex distribution were similar across all four groups. A higher proportion of patients had diabetes mellitus in the LTR group. However, the presence of comorbidities was comparable across all four groups. A total of 29 individuals (12.1 %) in the cohort had a history of COVID-19 infection > 6 months prior to enrollment. Seventy-five percent of individuals (n = 179) received ChAdOx1 nCoV-19 (Covishield) and 25 % (n = 61) received BBV152 (Covaxin) vaccines. All patients were tested for anti-spike antibody levels, while 18 in the healthy control, 12 in the non-cirrhosis CLD, 34 in the cirrhosis, and 15 in the LTR groups were assessed for cellular response. Of the sixty healthy controls who consented to the study, 12 were voluntary liver donors, 17 were health care workers, 15 were attendants of the CLD patients, and 16 were patients attending the outpatient department for non-specific gastrointestinal symptoms. The baseline characteristics are listed in Table 1 .
Fig. 1.
Consort chart. COVID-19, coronavirus disease; PCR, polymerase chain reaction. CLD, chronic liver disease; LTR, liver transplant recipient.
Table 1.
Baseline characteristic among the included individuals.
| Variables | Healthy controls (n = 60) | NCCLD (n = 50) | Cirrhosis (n = 113) | LTR (n = 17) | P |
|---|---|---|---|---|---|
| Age | 51.2 ± 8.75 | 49.34 ± 10.48 | 52.42 ± 9.93 | 51.41 ± 13.34 | 0.35 |
| Females | 22 (36.7 %) | 16 (32 %) | 24 (21.2 %) | 3 (17.6 %) | 0.1 |
| Comorbidities | |||||
| Diabetes mellitus | 19 (31.7 %) | 18 (36 %) | 54 (47.8 %) | 10 (58.8 %) | 0.07 |
| Hypertension | 15 (25 %) | 13 (26 %) | 39 (34.5 %) | 7 (41.2 %) | 0.38 |
| Hypothyroidism | 1 (1.7 %) | 1 (2 %) | 3 (2.7 %) | 0 | 0.89 |
| Ischemic heart disease | 1 (1.7 %) | 2 (4 %) | 6 (5.3 %) | 0 | 0.54 |
| Chronic pulmonary disease | 3 (5 %) | 0 | 1 (0.9 %) | 0 | 0.13 |
| Obesity (BMI > 25 kg/m2) | 20 (33.3 %) | 28 (56 %) | 53 (47 %) | 7 (41.2 %) | 0.11 |
| Malignancy|| | 1 (1.7 %) | 0 | 4 (3.5 %) | 2 (11.8 %) | 0.08 |
| History of COVID-19 infection (>6months prior) | 4 (6.7 %) | 6 (12 %) | 14 (12.4 %) | 5 (29.4 %) | 0.09 |
| Type of vaccine | |||||
| Covishield | 42 (70 %) | 34 (68 %) | 90 (79.3 %) | 13 (76.5 %) | 0.33 |
| Covaxin | 18 (30 %) | 16 (32 %) | 23 (20.4 %) | 4 (23.5 %) | |
| Time gap between two doses (days) | 55.27 ± 23.2 | 55 ± 25.1 | 54.9 ± 24.1 | 41.24 ± 15.84 | 0.15 |
| Time from last dose to vaccination (days) | 26.48 ± 9.26 | 23.92 ± 7.3 | 25.22 ± 8.37 | 25 ± 8.55 | 0.46 |
| Etiology of liver disease | |||||
| ARLD | 0 | 28 (24.8 %) | |||
| NAFLD/NASH | 24 (48 %) | 42 (37.2 %) | |||
| Hepatitis B | 20 (40 %) | 15 (13.3 %) | |||
| Hepatitis C | 2 (4 %) | 8 (7.1 %) | |||
| Others¶ | 2 (4 %) | 18 (15.92 %) | |||
| HBV/HCV + NASH | 2 (4 %) | 2 (1.8 %) | |||
| Hemoglobin (g/dL) | 13.35 ± 2.06 | 13.62 ± 1.97 | 11.72 ± 1.95 | 11.32 ± 1.55 | <0.001 |
| Total leukocyte counts (cells/mm3) | 7183.34 ± 1967.8 | 7555 ± 2153.31 | 6635.4 ± 6093.1 | 6105.88 ± 1544.53 | 0.51 |
| Platelet counts (lakhs/mm3) | 2.4 ± 1.1 | 2.44 ± 0.76 | 1.56 ± 1.03 | 1.93 ± 0.51 | <0.001 |
| Blood urea (mg/dl) | 24.06 ± 9.87 | 21.56 ± 5.5 | 27.38 ± 12.02 | 24.41 ± 6.2 | 0.006 |
| Serum creatinine (mg/dl) | 0.81 ± 0.16 | 0.82 ± 0.15 | 0.88 ± 0.25 | 0.89 ± 0.18 | 0.09 |
| Serum sodium (meq/dl) | 137.43 ± 3.2 | 137.32 ± 2.6 | 136.16 ± 3.44 | 137.35 ± 2.26 | 0.03 |
| INR | 1.1 ± 0.3 | 1.19 ± 0.32 | 1.32 ± 0.31 | 1.23 ± 0.34 | <0.001 |
| Total bilirubin (mg/dl) | 1.12 ± 0.7 | 1.06 ± 0.68 | 3.1 ± 4.71 | 1.01 ± 0.6 | <0.001 |
| Serum albumin (g/dl) | 4.41 ± 0.7 | 4.47 ± 0.87 | 3.58 ± 0.61 | 4.06 ± 0.53 | <0.001 |
| MELD score | 13.25 ± 5.57 | ||||
| Child-Pugh class (A/B/C) | 68/32/13 |
ARLD, alcohol-related liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; || Healthy control individual had history of thyroid malignancy treated 8 years back; four patients in cirrhosis group had hepatocellular carcinoma which were treated with locoregional therapy and two liver transplant recipients had HCC prior to liver transplantation. ¶Others includes autoimmune hepatitis, Wilsons disease, hepatic venous outflow tract obstruction and cryptogenic cause of CLD.
4.1. Immune response among patients with non-cirrhosis CLD
Most common cause of non-cirrhosis CLD was NAFLD (48 %), followed by viral hepatitis (40 %). The median anti-spike antibody levels among non-cirrhosis CLD (251 [3.8–4220] AU/mL) and healthy control group (208.5 [3.8–3950] AU/mL) were similar (P = 0.45). The proportion of non-responders were similar among non-cirrhosis CLD (n = 8; 16 %) and healthy control (n = 5; 8.3 %; P = 0.21) groups. CD4-central memory cells were higher, and CD4-effector cells were lower in the non-cirrhosis CLD patients. The percentage of B cells was also lower in the non-cirrhosis CLD group but not significant (Table 2 ) (Fig. 2 A and B).
Table 2.
Comparison of cellular immune response among healthy controls, non-cirrhosis chronic liver disease patients, compensated cirrhosis, decompensated cirrhosis, and liver transplant recipients.
| Cells | Healthy controls (n = 18) | Non-Cirrhosis CLD (n = 12) | P | Comp. Cirrhosis CLD (n = 19) | Decomp. Cirrhosis (n = 15) | P (post-hoc comparison) | LTR (15) | P |
|---|---|---|---|---|---|---|---|---|
| CD3% | 60.46 ± 19.18 | 64.55 ± 15.76 | 0.54 | 60.15 ± 13.02 | 62.2 ± 15.36 | 0.92 | 64.22 ± 13.2 | 0.52 |
| CD4% | 46.58 ± 15.38 | 49.91 ± 9.7 | 0.51 | 49.6 ± 18.15 | 48.14 ± 14.2 | 0.85 | 52.44 ± 10.38 | 0.21 |
| CD4 Central memory% | 37.01 ± 14.72 | 48.63 ± 15.18 | 0.04 | 37.8 ± 10.65 | 45.52 ± 10.97 | 0.1 | 42.5 ± 12.84 | 0.26 |
| CD4 Naïve% | 20.11 ± 9.41 | 15.97 ± 7.74 | 0.21 | 21.64 ± 14.95 | 11.76 ± 9.92 | 0.04 (HC vs CC-0.69; HC vs DC-0.05 CC vs DC-0.02) | 32.26 ± 13.64 | 0.005 |
| CD4 effector memory% | 28 ± 6.62 | 26.25 ± 7.61 | 0.51 | 33.03 ± 14.82 | 37.53 ± 8.52 | 0.05 (HC vs CC-0.16; HC vs DC-0.01 CC vs DC-0.23) | 21 ± 13.34 | 0.058 |
| CD4 effector % | 16.81 ± 9.7 | 9.1 ± 6.51 | 0.02 | 9.89 ± 11.5 | 4.68 ± 4.1 | 0.002 (HC vs CC-0.03; HC vs DC-<0.001 CC vs DC-0.1) | 3.86 ± 1.89 | <0.001 |
| CD8% | 34.78 ± 13.17 | 38.03 ± 10.62 | 0.47 | 38.22 ± 17.05 | 41.32 ± 14.78 | 0.47 | 40.78 ± 10.46 | 0.16 |
| CD8 CENTRAL MEMORY% | 17.82 ± 8.18 | 18.62 ± 10.16 | 0.81 | 15.23 ± 7.04 | 23.13 ± 11.22 | 0.04 (HC vs CC-0.37; HC vs DC-0.09 CC vs DC-0.01 | 21.62 ± 14.43 | 0.35 |
| CD8NAIVE% | 24.41 ± 8.33 | 21.83 ± 5.52 | 0.35 | 28.88 ± 14.2 | 21.53 ± 4.64 | 0.11 | 43.94 ± 20.31 | 0.001 |
| CD8 EFFECTOR MEMORY% | 18.47 ± 8.36 | 14.45 ± 7.65 | 0.19 | 18.94 ± 9.92 | 18.97 ± 6.26 | 0.98 | 16.61 ± 8.74 | 0.53 |
| CD8 EFFECTOR% | 39.22 ± 8.82 | 45.16 ± 12.91 | 0.14 | 36.95 ± 13.18 | 36.34 ± 10.45 | 0.72 | 17.8 ± 11.34 | <0.001 |
| B CELLS% | 16.23 ± 9.33 | 11.14 ± 4.03 | 0.08 | 21.15 ± 10.53 | 10.86 ± 7.61 | 0.01 (HC vs CC-0.11; HC vs DC-0.11 CC vs DC-0.003) | 11.11 ± 6.8 | 0.09 |
| B MEMORY CELLS % | 3.43 ± 1.46 | 3.36 ± 1.42 | 0.9 | 3 ± 1.25 | 2.34 ± 1.24 | 0.08 (HC vs CC-0.32; HC vs DC-0.02 CC vs DC-0.16) | 4.08 ± 1.7 | 0.25 |
CLD, chronic liver disease; CC, compensated cirrhosis; DC, decompensated cirrhosis; HC, healthy controls; LTR, liver transplant recipients.
P is in comparison with healthy control group.
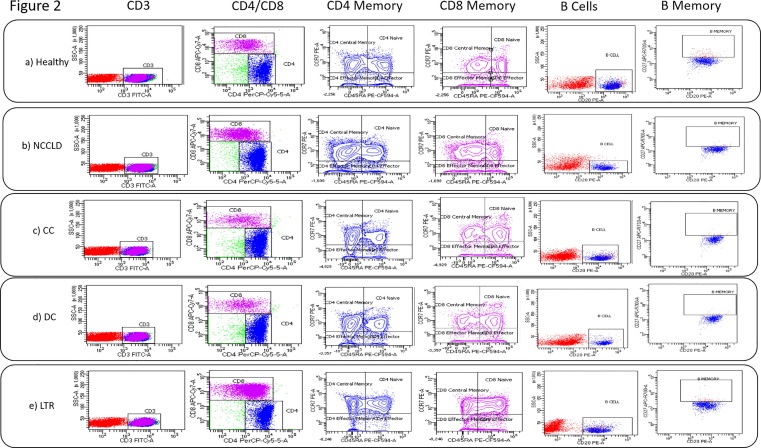
Fig. 2.
FACS plots showing gating strategy in PBMC samples of Healthy (a), non-cirrhosis CLD (b), compensated cirrhosis (c), decompensated cirrhosis (d), and liver transplant recipients (e) for T and B lymphocyte memory markers. T lymphocytes CD4 and CD8 were gated for central memory (CCR7 + CD45RA-), naïve (CCR7 + CD45RA + ), effector memory (CCR7-CD45RA-), and effector (CCR7-CD45RA + ) phenotype. B lymphocytes (CD20 + ) were gated for B Memory (CD27 + ) phenotype. Flow cytometry data were analyzed using BD Facs Diva software. PBMC, peripheral blood mononuclear cells; CD, cluster of differentiation; CLD, chronic liver disease.
4.2. Immune response among cirrhosis patients
A total of 113 patients had cirrhosis. Twenty-five percent (n = 28) in the cirrhosis group were non-responders compared to only 8.3 % in the healthy control group (P = 0.009). Despite having higher CD4-effector memory cells, CD4-effector cells were lower in the cirrhosis population than in the healthy control (Supplementary Table 1).
4.3. Sub-group analysis of cirrhosis population
Of the 113 patients, 63 and 50 were compensated and decompensated cirrhosis, respectively. The most common cause of cirrhosis was NASH in 37.2 % of patients, followed by ARLD in 24.8 %. Of the 50 patients with decompensated cirrhosis, five patients had a history of acute variceal bleeding and belonged to Child-Pugh class A, 32 patients were in Child-Pugh class B, and 13 were in Child-Pugh class C. The mean MELD NA score was 13.25 ± 5.57 among decompensated cirrhosis patients.
A higher proportion of patients in decompensated cirrhosis group (n = 17; 34 %) were non-responders than those in the compensated cirrhosis (n = 11; 17.5 %; P = 0.04) and healthy control (n = 5; 8.3 %; P = 0.001) groups. Non-response was similar among the healthy control and compensated cirrhosis group (P = 0.18). The proportion of immune cells was similar among both compensated cirrhosis and healthy control groups except for CD4-effector cells, which was lower in compensated cirrhosis group. In the decompensated cirrhosis group, 31.3 % (n = 10) in Child-Pugh class B and 54 % (n = 7) in Child-Pugh class C were non-responders. The antibody level in compensated cirrhosis group was 286 (3.88–4220) AU/mL compared to 121.5 (3.88–4220) AU/mL in decompensated cirrhosis group and 208.5 (3.88–3950) AU/mL in healthy control group (P = 0.56). CD4-naïve cells, CD4-effector cells, B cells, and B-memory cells were lower in the decompensated cirrhosis group than in the healthy control group. Though the central memory cells were higher in the decompensated cirrhosis group, they could not differentiate into effector cells (Table 2) (Fig. 2C and 2D).
4.4. Characteristics of LTR
All patients had undergone living donor liver transplantation (LDLT). The time from liver transplantation to inclusion in the study was 15.82 ± 10.17 months. Most common indication for LDLT was ARLD in eight, NASH in four, HBV in three, and HVOTO in two patients. All 17 patients were on calcineurin inhibitors (tacrolimus). Twelve patients were on mycophenolate mofetil (anti-metabolite). Five and two patients were on mechanistic target of rapamycin inhibitors (mTORi, everolimus) and corticosteroid therapy, respectively. The mean trough levels of tacrolimus was 5.76 ± 2.66 ng/mL.
4.5. Humoral response
Ten patients in LTR group (58.8 %) had antibody levels < 15 AU/mL compared to only five patients (8.3 %) in the healthy control group (p < 0.001). The antibody levels were lower in the LTR group (3.8 [3.8–978] AU/ml vs 208.5 [3.8–3950] AU/ml in the healthy group; P = 0.03). Seventy percent of those on MMF were non-responders. All the patients on everolimus (mTORi) were non-responders, of whom three were also on MMF.
4.6. Cellular response
CD4- and CD8-naïve cells were higher in the marrow in the LTR group, while the CD4-effector memory cells and CD4- and CD8-effector cells were lower in the LTR group. Furthermore, the proportion of B cells was lower in the LTR group than in the healthy control group (Table 2) (Fig. 2E).
4.7. Incidence of breakthrough infections post-vaccination
The cohort had 13 breakthrough infections (3-post Covaxin and 10-post covishield). The mean time from vaccination to infection was 4.31 ± 2.78 weeks. The median antibody levels in these 13 patients were 149 (7.8–3950) AU/ml. Only one patient had antibody levels < 15 AU/ml (non-responder). The incidence of breakthrough infections was 3.3 % (n = 2) in the healthy control group, 2 % (n = 1) in the non-cirrhosis CLD group, 8 % (n = 9; five in compensated cirrhosis and four in decompensated cirrhosis) in cirrhosis group, and 6 % (n = 1) in LTR group. All breakthrough infections were mild except in two patients (etiology: AIH and NASH) in the decompensated cirrhosis group who developed moderate disease requiring hospitalization. The patient with NASH was treated with remdesivir and required oxygen therapy, while the other recovered with conservative management.
4.8. Sub-group analysis comparing those with a history of infection (>6 months before inclusion)
A total of 29 individuals (12.1 %) in the cohort had a history of COVID-19 infection > 6 months before enrollment. Four, six, fourteen, and five in the healthy control, non-cirrhosis CLD, cirrhosis, and LTR groups had a history of infection. All the infections were mild and had not required hospitalization. The proportion of non-responders was similar among patients with a history of infection (10.3 %; n = 3/29) and those without a history of infection (22.7 %; 48/211; P = 0.12). Antibody levels were higher in patients with a history of vaccination (430 [3.8–3810] vs 149 [3.8–4220]; P = 0.18 in those without a history of infection) but not significant.
4.9. Response based on the vaccine type
Of the 61 individuals who had received covaxin, 31.1 % (n = 19) were non-responders, and 18 % (n = 32) of the 179 individuals who had received covishield were non-responders (P = 0.03). The non-response among all groups was similar in covaxin group. However, a higher proportion of patients in decompensated cirrhosis and LTR group than healthy control group were non-responders among those who received covishield. The antibody levels were lower in the covaxin group than in the covishield group. The summary of non-responders and antibody levels is depicted in Fig. 3 and detailed in supplementary table 2.
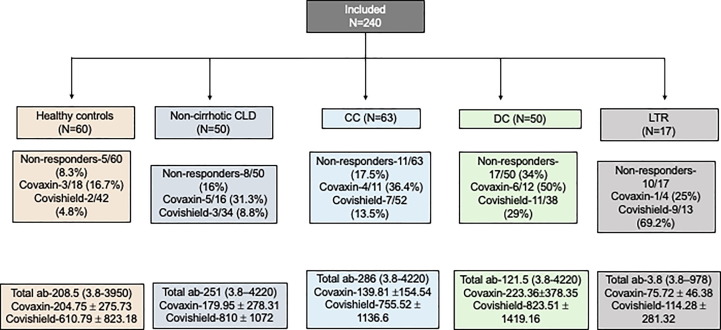
Fig. 3.
Summary of non-responders and antibody levels. CLD, chronic liver disease; CC, compensated cirrhosis; DC, decompensated cirrhosis; LTR, liver transplant recipients; ab, antibody.
5. Discussion
This novel study demonstrates that patients with decompensated cirrhosis and LTRs have poor humoral and cellular responses after ChAdOx1 nCoV-19 (recombinant type) and BBV152 (inactivated type) vaccination. Patients with compensated cirrhosis and non-cirrhosis CLD have comparable humoral and cellular responses to healthy controls. Breakthrough infections are less frequent and mild following COVID-19 vaccination. The response was poorer in those who received the inactivated vaccine but similar across all groups.
Influenza and SARS-CoV-2 virus-related pneumonia are associated with high morbidity and mortality in patients with cirrhosis. [2], [16], [17], [18] Although vaccination does not prevent infection, all vaccines reduce the need for hospitalization, modify the course of the disease, and improve the short-term and long-term outcomes of viral pneumonia. [6], [8], [19], [20] Therefore, patients with cirrhosis should be vaccinated as a priority group to prevent and modify the course of vaccine-modifiable diseases, including COVID-19. [16], [21].
The efficacy of COVID-19 vaccines in patients with cirrhosis and LTR has been demonstrated to be low or variable. Due to the inherent altered immune response in patients with decompensated cirrhosis, the antibody response is more inadequate and ill-sustained than in healthy controls. [22] John et al. assessed the efficacy of mRNA vaccines in a large cohort of 40,074 patients with cirrhosis and demonstrated that even a single dose of mRNA vaccine could reduce the risk of COVID-19 infections by 65 % and provide 100 % protection against hospitalization and death due to COVID-19 infection. [5] However, the efficacy of the mRNA vaccine was lower in patients with decompensated cirrhosis, in whom only 50 % of patients could be prevented from COVID-19 infection. [5] Inactivated vaccines are safer and produce adequate immune responses among patients with chronic liver disease. [23], [24].
Thuluvath et al. reported similar non-response to mRNA vaccines among patients with cirrhosis (23 %) and non-cirrhosis CLD population (25 % poor responders). [11] However, they included only ten patients with decompensated cirrhosis which was a significant limitation. The present study demonstrates that patients with compensated cirrhosis and non-cirrhosis CLD patients generate similar humoral and cellular responses as healthy controls. However, pronounced cirrhosis-related immune dysfunction in decompensated cirrhosis patients may explain the lower levels of CD4-naïve cells and CD4-effector cells. In concurrence with lower antibody response, B cells were significantly lower in decompensated cirrhosis patients.
Liver transplant recipients are also poor responders to COVID-19 vaccines due to immunosuppression. Solid organ transplant recipients also demonstrate a poor T-cell response to mRNA vaccines. [25] Approximately 17 % of recipients develop adequate responses after the first dose of mRNA vaccines, and approximately 40–50 % develop an adequate antibody response after the second dose. [11], [12], [13] Poor response is usually reported among elderly patients and those receiving mycophenolate mofetil. [12] We noted that only 41.2 % of LTRs developed adequate antibody response after the second dose. LTRs had a significantly higher proportion of CD4- and CD8-naïve cells in bone marrow but considerably lower levels of effector cells, implying a lack of differentiation due to the immunosuppressive drugs. Furthermore, the lower number of B cells also explains the poor antibody response in these patients. Therefore, the need for vaccine boosters, COVID-19 surveillance, retesting COVID-19 seroprotection status, and heterologous vaccination needs further research.
We noted that inactivated vaccines elicited weak immune responses. Inactivated vaccines stimulate antibody-mediated immune responses, which have a shorter lifespan with weak immunogenicity. [26], [27] Inactivated viral vaccines induce low cytotoxic CD8+ cells, which are required for effective immune responses and greater and longer immunogenicity. [28] Though inactivated vaccines are safer than live attenuated vaccines, the immunogenic epitopes may deform structurally during the inactivation process, destabilizing the protection that must be delivered. [29] Several studies have reported that inactivated SARS-CoV-2 vaccines can induce adequate antibody response and reduce disease severity. [30], [31], [32] Despite a suboptimal immune response, inactivated vaccines provide satisfactory immunogenicity and protection from severe infections. [33], [34].
The incidence of breakthrough infections in healthy individuals is reported to be 2.6 % after mRNA vaccination. [15] We noted that the incidence of breakthrough infections in our cohort was similar to that in previous reports among healthy controls and non-cirrhosis CLD patients. However, the incidence was slightly higher in patients with cirrhosis and LTRs. Moreover, both COVID-19 vaccines were found to be safe. As reported previously, none of the patients had adverse outcomes except two patients with decompensated cirrhosis who required hospitalization. [6], [7] The strain of COVID-19 varied throughout the pandemic. [35], [36], [37] During the study period, most patients were probably infected with Delta variant. [35], [36] However, this is difficult to ascertain as the number of breakthrough infections was few.
Limitations: Response to vaccines may not be uniform in each individual, and this study may not be a true representative of the whole population at large. Secondly, the methods used to assess the antibody and cellular response are not the gold standards. However, LIAISON® XL is approximately 95 % in agreement with PRNT, which is the gold standard test. Nevertheless, the major strengths of this study are the prospective collection of data, considerably large sample size, and comparison of both cellular and humoral responses to both vector-based and inactivated virus vaccines.
In conclusion, patients with non-cirrhosis CLD and healthy controls have a comparable response to recombinant (ChAdOx1 nCoV-19) and inactivated vaccines (BBV152). Patients with cirrhosis especially decompensated cirrhosis and liver transplant recipients, exhibit poor humoral and cellular immune responses to the available COVID-19 vaccines. Recent studies have demonstrated that a booster dose may enhance the antibody responses in these immunocompromised patients. [38] Therefore, a booster dose is recommended for all patients depending on the COVID-19 seroprotective levels.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
None.
Disclosures
Nothing to disclose.
Data transparency statement
Data will be shared on request to the corresponding author.
Preprint server
None.
Grant support
Asian Healthcare Foundation, India.
Specific author contributions
Concept by AVK and PNR; data acquisition by BAG, SS, SI, and AVK; initial drafting by AVK and SJ; statistical analysis by AVK and MP; Figures by AVK and SJ; laboratory support by SJ, VS, DG, VKV, and MS; administrative support by PNR and DNR; critical analysis and final editing by AVK, DNR, KRR, and PNR. All authors approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Bajaj J.S., Garcia-Tsao G., Biggins S.W., Kamath P.S., Wong F., McGeorge S., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar P., Sharma M., Sulthana S.F., Kulkarni A., Rao P.N., Reddy D.N. Severe Acute Respiratory Syndrome Coronavirus 2-related Acute-on-chronic Liver Failure. J Clin Exp Hepatol. 2021;11(3):404–406. doi: 10.1016/j.jceh.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni A.V., Tevethia H.V., Premkumar M., Arab J.P., Candia R., Kumar K., et al. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb G.J., Marjot T., Cook J.A., Aloman C., Armstrong M.J., Brenner E.J., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John B.V., Deng Y., Scheinberg A., Mahmud N., Taddei T.H., Kaplan D., et al. Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern Med. 2021;181(10):1306. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon A.M., Webb G.J., García-Juárez I., Kulkarni A.V., Adali G., Wong D.K., et al. SARS-CoV-2 Infections Among Patients With Liver Disease and Liver Transplantation Who Received COVID-19 Vaccination. Hepatol Commun. 2022;6:889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John B.V., Deng Y., Schwartz K.B., Taddei T.H., Kaplan D.E., Martin P., et al. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76(1):126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John BV, Deng Y, Khakoo NS, Taddei TH, Kaplan DE, Dahman B. Coronavirus Disease 2019 Vaccination Is Associated With Reduced Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Death in Liver Transplant Recipients. Gastroenterology. 2022;162:645-7 e2. [DOI] [PMC free article] [PubMed]
- 9.Fix O.K., Blumberg E.A., Chang K.-M., Chu J., Chung R.T., Goacher E.K., et al. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74(2):1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325(17):1784. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuss I.J., Kanof M.E., Smith P.D., Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. 2009;85(1) doi: 10.1002/0471142735.im0701s85. [DOI] [PubMed] [Google Scholar]
- 15.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni A.V., Premkumar M., Arab J.P., Kumar K., Sharma M., Reddy N.D., et al. Early Diagnosis and Prevention of Infections in Cirrhosis. Semin Liver Dis. 2022;42(03):293–312. doi: 10.1055/a-1869-7607. [DOI] [PubMed] [Google Scholar]
- 17.Schütte A., Ciesek S., Wedemeyer H., Lange C.M. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70(4):797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni A.V., Parthasarathy K., Kumar P., Sharma M., Reddy R., Chaitanya Akkaraju Venkata K., et al. Early liver transplantation after COVID-19 infection: The first report. Am J Transplant. 2021;21(6):2279–2284. doi: 10.1111/ajt.16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Härmälä S., Parisinos C.A., Shallcross L., O'Brien A., Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. 2019;9(9):e031070. doi: 10.1136/bmjopen-2019-031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni A.V., Khelgi A., Sekaran A., Reddy R., Sharma M., Tirumalle S., et al. Post Covid-19 Cholestasis: A Case Series and Review of Literature. J Clin Exp Hepatol. 2022 doi: 10.1016/j.jceh.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekpanyapong S., Reddy K.R. Infections in Cirrhosis. Curr Treat Options Gastroenterol. 2019;17(2):254–270. doi: 10.1007/s11938-019-00229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willuweit K., Frey A., Passenberg M., Korth J., Saka N., Anastasiou O.E., et al. Patients with Liver Cirrhosis Show High Immunogenicity upon COVID-19 Vaccination but Develop Premature Deterioration of Antibody Titers. Vaccines (Basel) 2022;10(3):377. doi: 10.3390/vaccines10030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He T., Zhou Y., Xu P., Ling N., Chen M., Huang T., et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022;42(6):1287–1296. doi: 10.1111/liv.15173. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Hou Z., Liu J., Gu Y.e., Wu Y., Chen Z., et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol. 2021;75(2):439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schramm R., Costard-Jäckle A., Rivinius R., Fischer B., Müller B., Boeken U., et al. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol. 2021;110(8):1142–1149. doi: 10.1007/s00392-021-01880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Yin S., Tong X., Tao Y., Ni J., Pan J., et al. Dynamic SARS-CoV-2-specific B-cell and T-cell responses following immunization with an inactivated COVID-19 vaccine. Clin Microbiol Infect. 2022;28(3):410–418. doi: 10.1016/j.cmi.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng Z., Ma D., Duan S., Zhang J., Yue R., Li X., et al. Immunological Study of Combined Administration of SARS-CoV-2 DNA Vaccine and Inactivated Vaccine. Vaccines (Basel) 2022;10(6):929. doi: 10.3390/vaccines10060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y.D., Chi W.Y., Su J.H., Ferrall L., Hung C.F., Wu T.C. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci. 2020;27:104. doi: 10.1186/s12929-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang T., Liang B., Wang H., Quan X., He S., Zhou H., et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell Mol Immunol. 2021;18(12):2679–2681. doi: 10.1038/s41423-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sripongpun P., Pinpathomrat N., Bruminhent J., Kaewdech A. Coronavirus Disease 2019 Vaccinations in Patients With Chronic Liver Disease and Liver Transplant Recipients: An Update. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.924454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aleem A., Akbar Samad A.B., Slenker A.K. Treasure Island (FL): StatPearls Publishing Copyright © 2022. StatPearls Publishing LLC; 2022. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). StatPearls. [Google Scholar]
- 36.Munigela A, Sowpati DT, M S, Banu S, Siva AB, V JK, et al. Clinical outcomes in Covishield (ChAdOx1) and Covaxin (BBV-152) vaccinated individuals hospitalized with the Delta variant (B.1.617.2). IJID Reg. 2022. [DOI] [PMC free article] [PubMed]
- 37.Kulkarni AV, Metage CS, Gora BA, Tirumalle S, Rakam K, Satyavadi A, et al. SARS-CoV-2 Omicron variant infection was associated with higher morbidity in patients with cirrhosis. Gut. 2022:gutjnl-2022-328451. [DOI] [PubMed]
- 38.Chauhan M., Nzeako I., Li F., Thuluvath P.J. Antibody response after a booster dose of SARS-CoV-2 vaccine in liver transplant recipients and those with chronic liver diseases. Ann Hepatol. 2022;27(4) doi: 10.1016/j.aohep.2022.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.