Fig. 2.
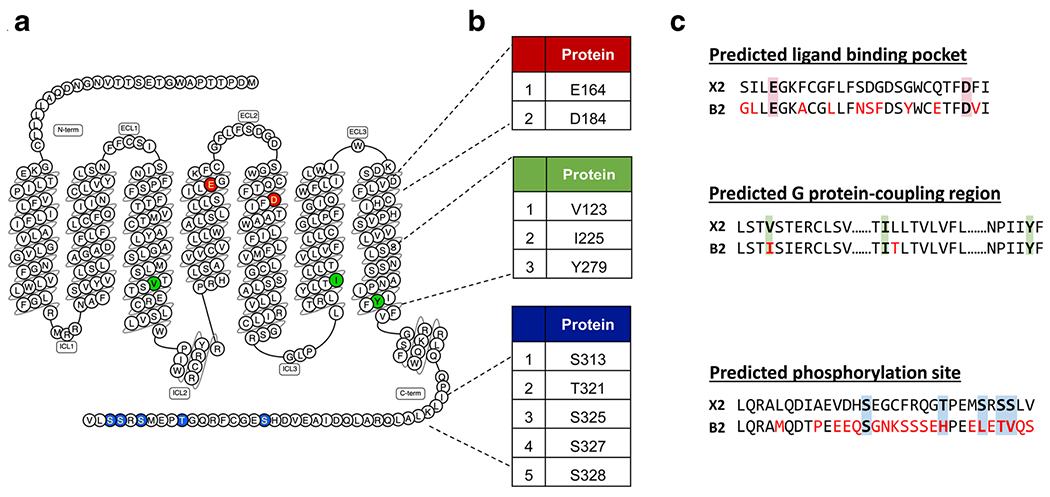
Predicted ligand binding, G protein coupling, and phosphorylation regions of MRGPRX2 with sequence comparisons to its mouse ortholog MrgprB2. a Snake diagram of secondary structure of MRGPRX2 with solid background denoting residues responsible for ligand binding (red), G protein coupling (green), and phosphorylation (blue) of MRGPRX2. b Amino acid numbering for each MRGPRX2 residues. c Site-specific pairwise sequence alignment of human MRGPRX2 (top) with mouse MrgprB2 (bottom) at predicted ligand binding (red background), G protein-coupling (green background), and phosphorylation regions (blue background). Red font color indicates different residues in MrgprB2 when compared to MRGPRX2
