Coronary obstruction remains a pernicious complication of transcatheter aortic valve replacement (TAVR). It is caused by outward displacement of a valve leaflet, directly blocking the coronary ostium or sealing the coronary sinus. Bioprosthetic or native aortic leaflet intentional laceration to prevent coronary artery obstruction (BASILICA) creates a slit lesion along the midline of the aortic leaflet, which splays after TAVR, allowing coronary perfusion. Despite successful BASILICA, a small fraction of patients experience some degree of obstruction, such as from a prolapsing leaflet. We developed a leaflet removal technique (CATHeter Electrosurgical Debulking and RemovAL [CATHEDRAL]; Video 1) to treat such patients and herein report the first application.
An 82-year-old woman with coronary artery bypass graft surgery and surgical aortic valve replacement (3-F × 23-mm ATS [ATS medical]) 11 years ago, recent left main percutaneous coronary intervention, and cirrhosis was referred for TAVR. Multiphase computed tomography (Figures 1A and 1B) revealed a long left coronary leaflet that prolapsed dynamically, causing severe aortic regurgitation (AR) (Figure 1C). Predicted valve-to-sinotubular junction distance was <2 mm, risking sinus sequestration.
FIGURE 1. Baseline CT and Key Procedure Steps to Set Up CATHEDRAL.
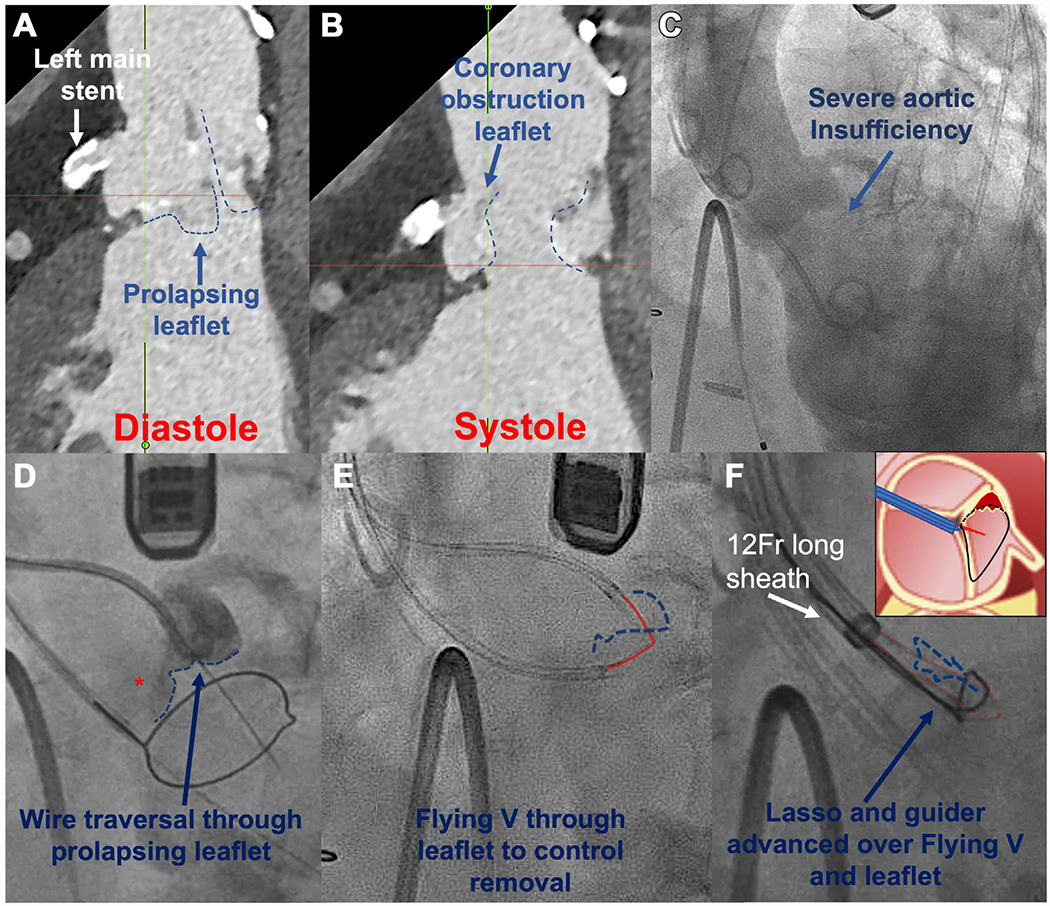
(A and B) Prolapsing left leaflet and coronary obstruction risk by long leaflet. (C) Aortography, severe AR. (D) Prolapsing leaflet (asterisk) traversed with AstatoXS20 wire. (E) Flying V across leaflet. (F) 12-F sheath and guider with snare lasso advanced over Flying V and energized. (F) Inset: The snare is advanced over the “corner” of the leaflet torn from the commissure. With traction and rotation of the Flying V (red lines), the remaining part of the leaflet can be pulled into the snare. Dashed blue lines indicate the leaflet.
AR = aortic regurgitation; CT = computed tomography; CATHEDRAL = Catheter electrosurgical debulking and removal.
CATHEDRAL entails grasping the prolapsing bioprosthetic leaflet with a guidewire and then enveloping and detaching the leaflet. The technique resembles BASILICA in that the leaflet was traversed with an energized Astato XS 20 0.014-inch guidewire (Asahi Intecc Medical) (Figure 1D), and a Flying V (guidewire kinked at midpoint) was advanced across the leaflet (Figure 1E), exteriorized through 2 guiding catheters. Unlike BASILICA, the Flying V was not focally denuded and was used, not for laceration, but to grasp the leaflet. The paired guiding catheters were replaced by a single 12-F sheath. Through the 12-F sheath and over the Flying V, a guiding catheter was inserted and delivered a loop snare around the base of the leaflet (Figure 1F). This patient had a leaflet that was prolapsing from a torn commissure; thus, we could get the snare around 1 “corner” of the leaflet and then with traction and rotation of the Flying V, pull the remainder of the leaflet into the snare (Figure 1F). After confirmation by transesophageal echocardiography, we tightened the snare, flushed dextrose solution, and energized the conductive lasso at 70 W “cut” mode. With simultaneous traction on the Flying V, the leaflet was detached from the surgical frame (Figure 2A). Because CATHEDRAL exacerbated AR with low diastolic pressure, unchanged systolic pressure, and left ventricular dilation, the TAVR was performed expeditiously. AR resolved immediately (Figure 2B) and the left coronary artery could be injected directly through the stent frame, demonstrating patency (Figure 2C). The total procedure time was 4.5 hours with 80 minutes of fluoroscopy. Patient convalescence was uneventful with discharge 2 days later and sustained improvements at 1-month follow-up.
FIGURE 2. Leaflet Removal.
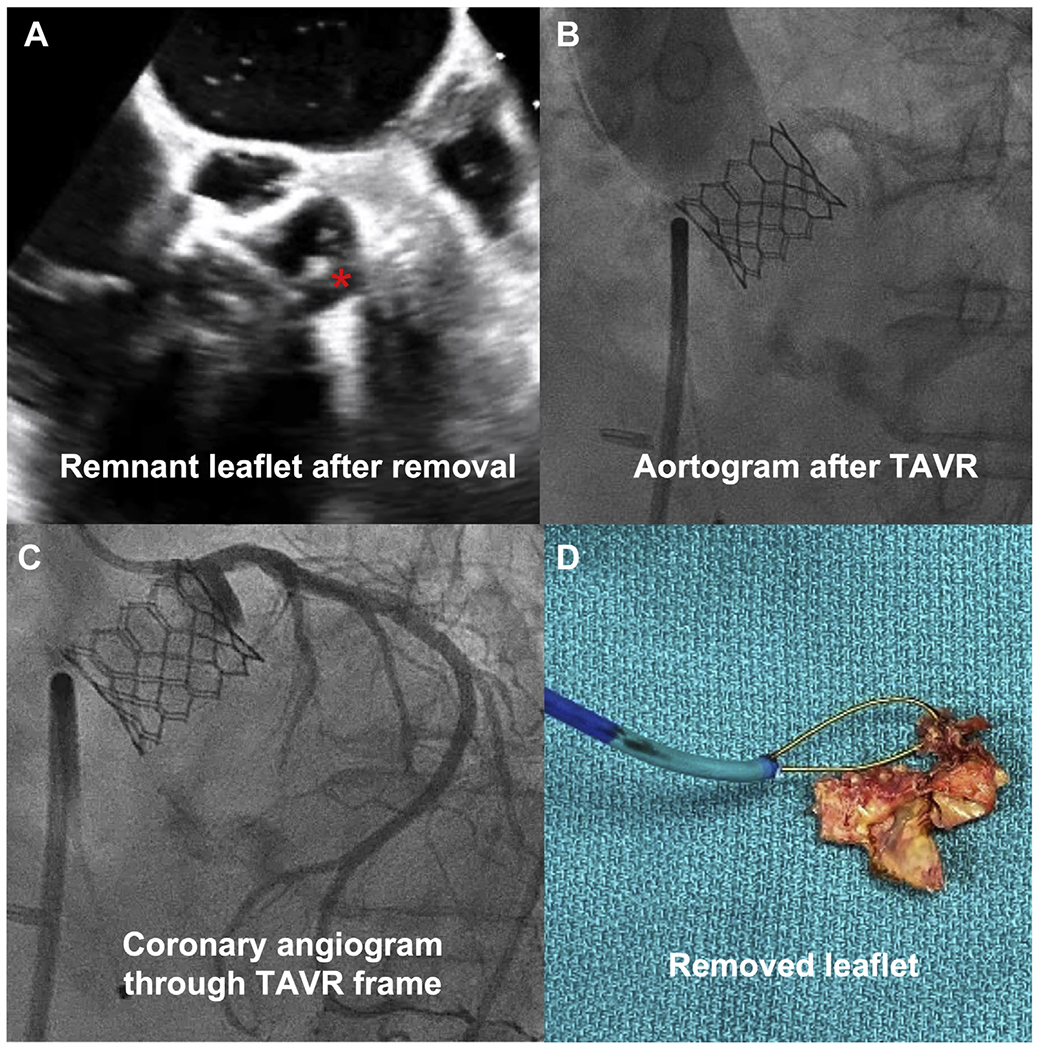
(A) Transesophageal echocardiography reveals small leaflet remnant (asterisk). (B) Transcatheter aortic valve replacement (TAVR) with no aortic regurgitation, patent coronary. (C) Coronary angiography through stent frame. (D) Gross specimen = aortic valve leaflet.
This report represents the first description to our knowledge of leaflet removal (Figure 2D) to facilitate TAVR. The crude, off-the-shelf tools described seem best suited to remove prolapsed surgical leaflets. With further refinements, the options to remove more-intact leaflets may extend the role of TAVR in lifetime management of valve disease.
Supplementary Material
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Supported by Emory Structural Heart and Valve program intramural funds and by National Institutes of Health (NIH) grant Z01-HL006040. Dr Babaliaros has institutional research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific; is a consultant for Edwards Lifesciences and Abbott Vascular; and has equity interest in Transmural Systems. Drs Gleason, Xie, and Byku have institutional research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr Khan is a proctor for Edwards Lifesciences and Medtronic; and is a coinventor on patents, assigned to the NIH, on devices for electrosurgical tissue laceration. Dr Bruce is a coinventor on patents, assigned to the NIH, on devices for electrosurgical tissue laceration. Dr Grubb is a consultant for Edwards Lifesciences and Medtronic; and has institutional research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr Paone has institutional research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific; and is a proctor for Edwards Lifesciences. Dr Lederman is a coinventor on patents, assigned to the NIH, on devices for electrosurgical tissue laceration. Dr Greenbaum has institutional research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific; is a proctor for Edwards Lifesciences and Medtronic; and has equity interest in Transmural Systems. Dr Rogers has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For a supplemental video, please see the online version of this paper.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


