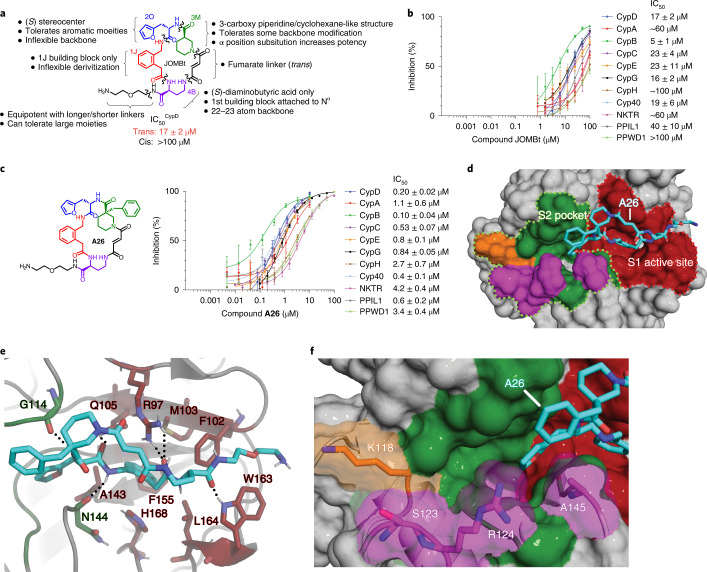
Fig. 1. Selection of a DNA-templated library of 256,000 macrocycles for CypD affinity reveals novel cyclophilin inhibitors.
a, Generalized trends in inhibition potency of CypD prolyl-isomerase activity from cyclophilin inhibition profiles of library hits and A-series macrocycles. b, JOMBt, showing weak and promiscuous cyclophilin inhibition of prolyl-isomerase activity. c, Compound A26 showing improved CypD potency but promiscuous inhibition. d, X-ray co-crystal structure of compound A26 (cyan) bound to CypD (PDB ID 7TGT, 1.06 Å resolution), shown as a space-filling model. A26 has a dual-binding mode involving the active site (red) and S2 pocket (green) of CypD. e, Active site binding interactions with A26. The phenyl group provides the primary hydrophobic interactions with F102, M103, A143, F155, L164, and H168. Black dashes show predicted hydrogen bonding interactions with R97, Q105, G114, N144, and W163 and the backbone of the A26. f, S2 pocket binding pose of the furan of A26, exhibiting a shallow interaction that does not engage non-conserved residues K118 (orange), S123 (magenta), and R124 (magenta) on the far side of the pocket. IC50 values reflect mean ± s.e.m. of three technical replicates. Data points and error bars reflect mean ± s.d. of individual assays at one dose.