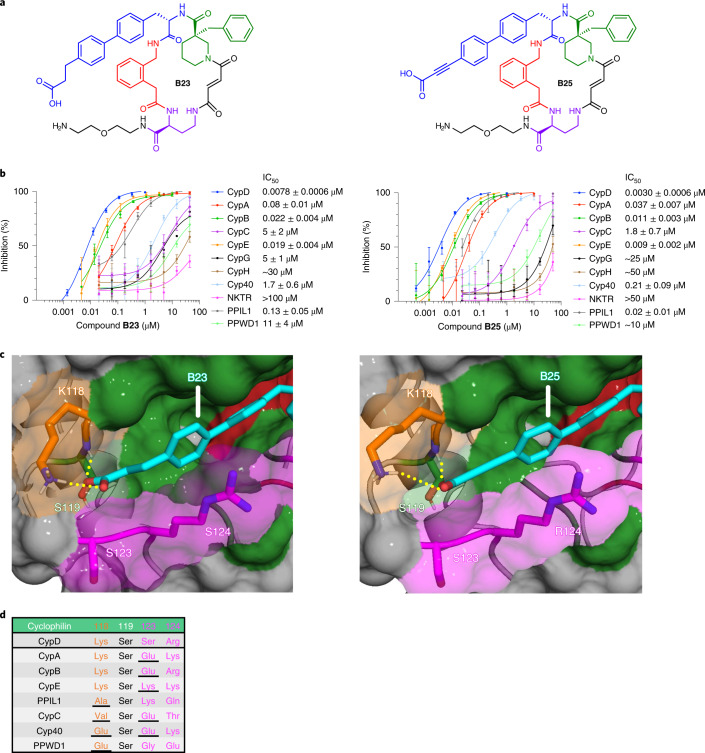
Fig. 3. Carboxylate-containing biphenyl derivatives of B2 offer enhanced CypD selectivity.
a, Structure of B23 and B25. b, Cyclophilin prolyl-isomerase inhibition screens for B23 and B25. c, Co-crystal structures of B23 (PDB ID 7TH7, 1.18 Å resolution) and B25 (PDB ID 7THC, 1.57 Å resolution) bound to CypD, viewing the S2 pocket. Active site binding is identical to that of A26 (Fig. 1e). Yellow dashes indicate predicted hydrogen bonds. d, List of residues on the far side of the S2 pocket of cyclophilins that are proximal to the ligand carboxylates, with important deleterious interactions underlined. The biphenyl group with 3-carbon carboxylates, B23 and B25, achieve strong selectivity over cyclophilins without a residue homologous to CypD K118. B23 and B25 show similar inhibition potencies for CypD and other cyclophilins that contain a Lys residue homologous to CypD K118, but slightly attenuated depending on the identity of residue 123. IC50 values reflect mean ± s.e.m. of three technical replicates. Data points and error bars reflect mean ± s.d. of individual assays at one dose.