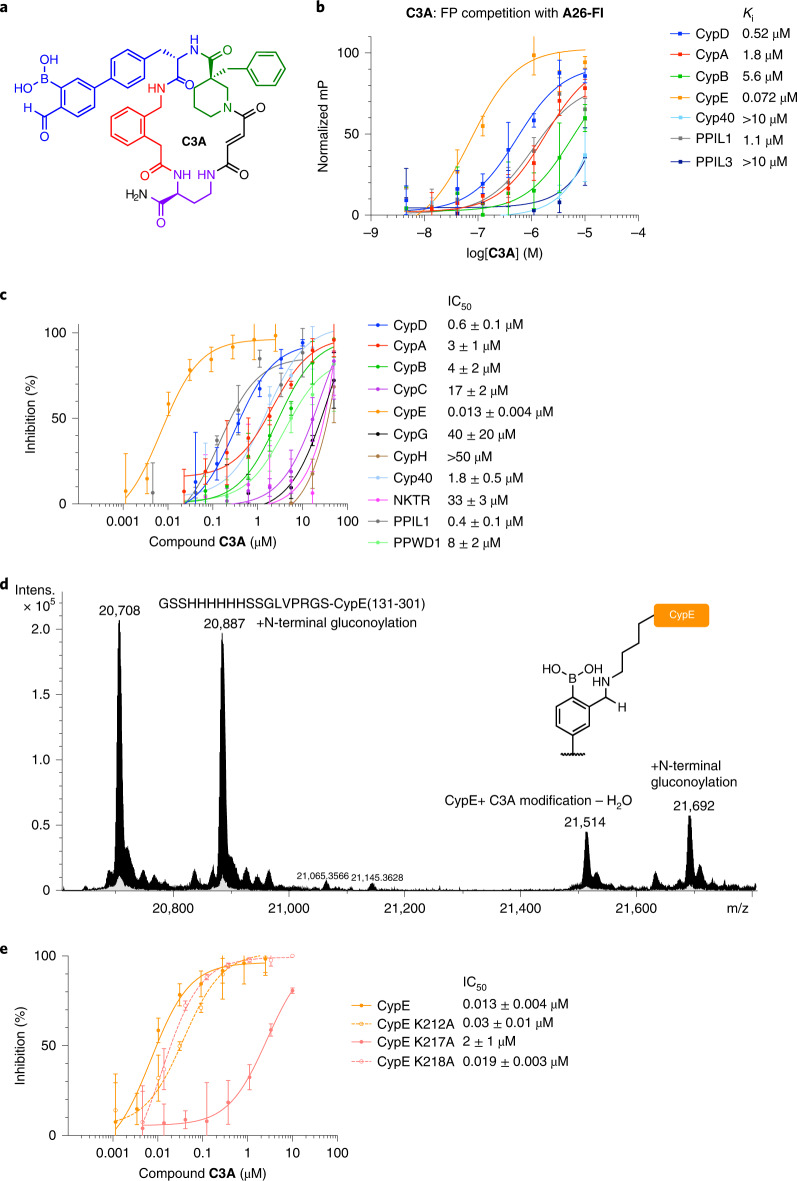
Fig. 6. Aryl-carbonyl boronic acid C3A achieves selective inhibition of CypE.
a, Structure of C3A. b, C3A fluorescence polarization (FP) competition with A26-Fl against cyclophilins with S2 pocket lysines. c, Cyclophilin prolyl-isomerase inhibition screen of C3A, showing potency and selectivity for CypE. d, Mass spectroscopy trace of CypE incubated with C3A and reduced with sodium cyanoborohydride. C3A shows an adduct consistent with CypE + amine–H2O (+806 Da), the result of iminoboronate formation followed by reductive amination. The mass of CypE is 20,708 Da, The CypE preparation also included N-terminal gluconoylation. e, Prolyl-isomerase inhibition by C3A against CypE S2 pocket lysine to alanine mutants. For the FP assay, the y-axis is normalized to internal control wells containing A26-Fl only (100%) and A26-Fl with cyclophilin (0%). Values reflect mean of three technical replicates and error bars reflect s.d. of individual assays at one dose. For the prolyl-isomerase assay in c and the CypE wild-type dose–response curve in e, IC50 values reflect mean ± s.d. of four independent replicates (each comprising three technical replicates). Graph shows a representative single independent replicate (Independent replicate 3 is shown, containing three technical replicates) with data points and error bars reflecting mean ± s.d. of individual assays at one dose. Further independent replicates are shown in Supplementary Fig. 28b. For the prolyl-isomerase assay in e, IC50 values for the CypE mutants reflect mean ± s.e.m. of three technical replicates, while data points and error bars reflect mean ± s.d. of individual assays at one dose.