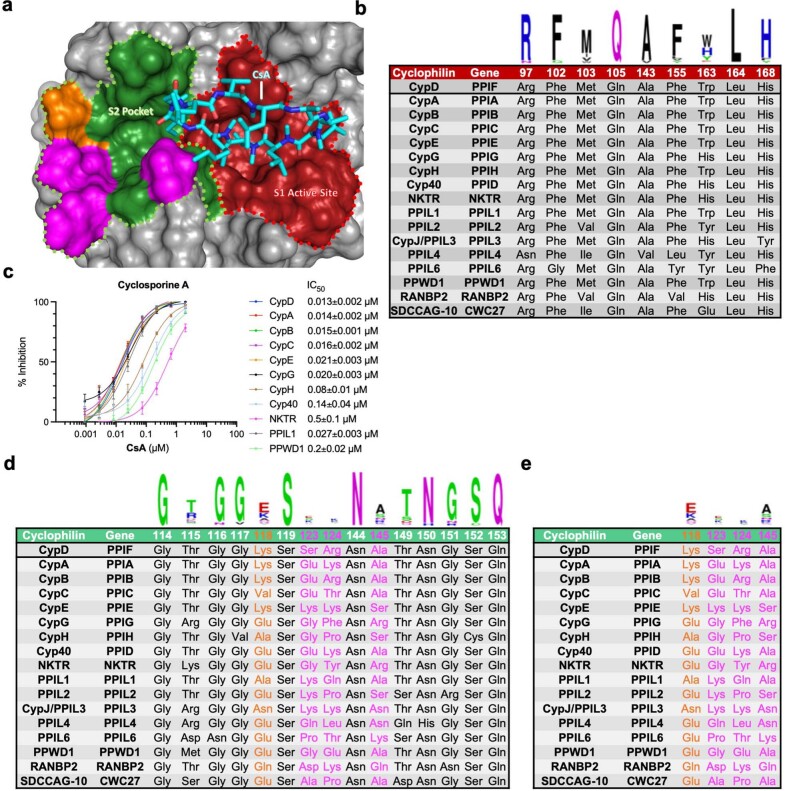
Extended Data Fig. 1. Exclusive active site binding results in promiscuous cyclophilin inhibition.
a, Co-crystal structure of CsA bound to CypD (PDB ID 2Z6W). CsA (cyan) binds to the active site (red) of CypD, a binding mode that does not engage more diversified residues that lie in the S2 pocket (green), including the primary gatekeepers (magenta) and the semiconserved K118 residue (orange). b, Active site (S1 pocket) residues with WebLogo plots, showing high conservation between the 17 cyclophilin isoforms with corresponding gene and protein identifier. c, Isomerization of the reporter peptide Suc-AAPF-AMC was measured with 5 nM cyclophilin and varying concentrations of CsA. CsA shows potent, but promiscuous inhibitory activity against all prolyl isomerase-active cyclophilins. Selective inhibition could rather be achieved through interactions with S2 pocket residues of cyclophilin subtypes (d). e, Cyclophilin S2 pocket residues that are the primary diversification sites within the active site-S2 pocket groove with WebLogo plots. Typical cyclophilin inhibitors such as CsA do not engage in interactions with these residues. All residue numbering is in reference to CypD. IC50 values reflect mean ± s.e.m. of three technical replicates. Data points and error bars reflect mean ± s.d. of individual assays at one dose.