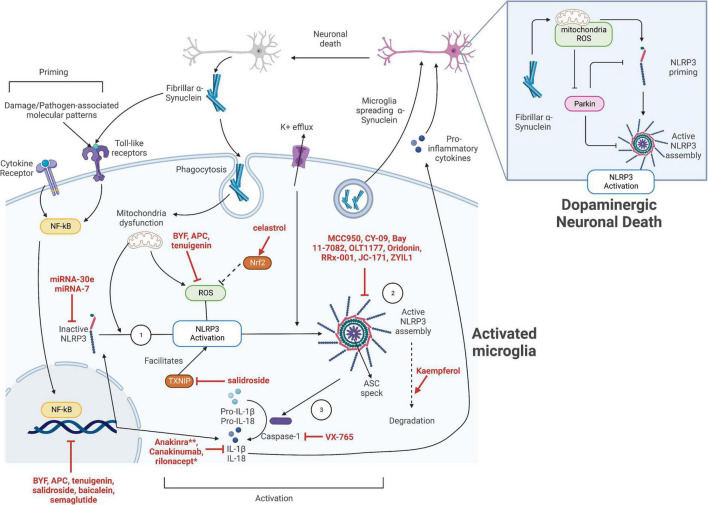
FIGURE 1.
The strategies for inhibition of NLRP3 inflammasome activation in Parkinson’s disease (PD). The activation of NLRP3 inflammasome in microglia can be divided into the priming stage and activation stage. Various inflammatory cytokines and damage-associated or pathogen-associated molecular patterns, including fibrillar α-synuclein, can activate the NF-κB pathway to upregulate the expression of NLRP3 sensor protein, pro-IL-1β, and pro-IL-18. The inactive NLRP3 proteins then oligomerize upon activation by the various stimuli including potassium ion efflux, mitochondrial dysfunction, and related reactive oxygen species (ROS) release. These signals might be generated by, but not limited to, the phagocytosis of fibrillar α-synuclein. The activated NLRP3 proteins then trigger an assembly of apoptosis-associated speck-like protein containing a CARD (ASC), which subsequently activate pro-caspase 1. Activated caspase 1 facilitates the maturation of pro-cytokines, leading to pro-inflammatory cytokine release. The released cytokines as well as fibrillar α-synuclein directly exocytosed by the microglia promotes neuronal death, leading to the release of more fibrillar α-synuclein, thus amplifying microglial activation and neuroinflammation. Potential target sites of NLRP3 activation in microglia include: ➀ targeting the NF-κB priming pathway to prevent the upregulation of NLRP3 and cytokines, however, this might be highly non-specific; ➁ directly targeting the inflammasome components and preventing its activation; ➂ targeting the downstream inflammasome effectors. The specific NLRP3 inhibitors mentioned in this work are indicated in red. **Anakinra and rilonacept indirectly inhibit IL-1β by binding to IL-1 receptors and serving as a decoy receptor for IL-1β, respectively.