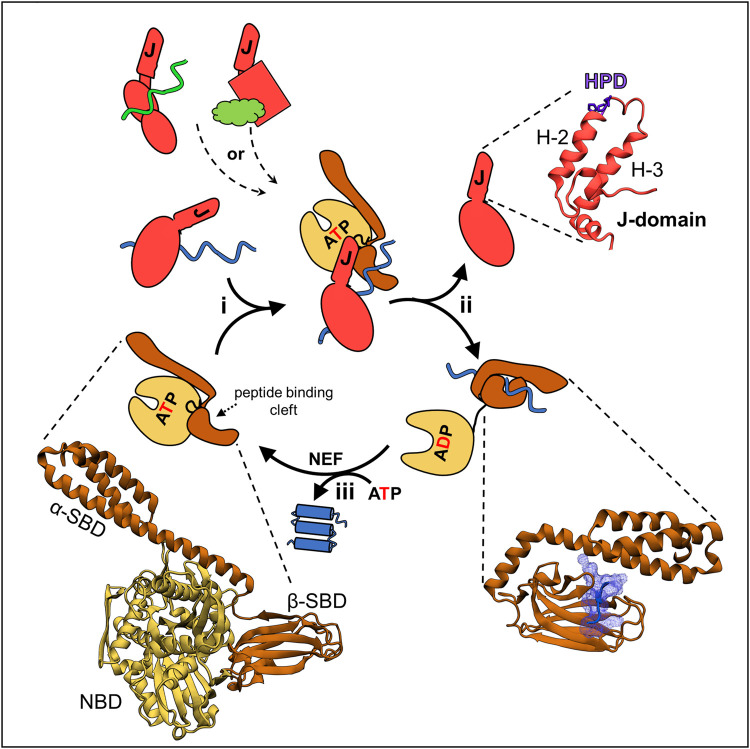
FIGURE 1.
JDP/Hsp70 client binding cycle and structures of key components. (upper left) Different JDPs (red) can deliver different clients, indicated by different colors and shapes, to the same Hsp70 by interacting with them directly via distinct client binding domains, indicated by different shapes. (center) This scheme depicts the cycle involved in folding of a client protein. The same cycle occurs when JDP/Hsp70 systems are involved in other cellular processes, including FeS cluster biogenesis (see Figure 3): (i) JDP-client complex interacts with ATP bound Hsp70. In this “open” conformation the nucleotide binding domain (NBD, beige) is docked on to the substrate binding domain (SBD, brown) and the interdomain flexible linker (black) is bound in a crevice of the NBD. This conformation allows easy access of client to the peptide binding cleft of β-SBD, as α-SBD is retracted from it, and enables binding of the J-domain, which interacts at the NBD-β-SBD interface. It also allows simultaneous, transient interactions of the J-domain and JDP-bound client, and thus synergistic stimulation of ATP hydrolysis by Hsp70. (ii) ATP hydrolysis triggers conformational changes resulting in separation of the NBD and SBD, only connected by a flexible linker. The α-SBD subdomain covers the peptide binding cleft of β-SBD, stabilizing its interaction with the client. Separation of the SBD and NBD also results in loss of the J-domain binding face, and thus JDP release. (iii) ADP exchange for ATP, often involving a nucleotide exchange factor (NEF), reverting Hsp70 to the ATP bound state, causing client release. Structures of critical components of the cycle from E. coli DnaK and DnaJ—the best studied JDP/Hsp70 system—are depicted (bottom left) the ATP bound conformation of DnaK (PDB ID: 4B9Q). (Upper right) J-domain architecture is conserved with helices 2 and 3 (H-2, H-3) forming a finger like structure. Loop between these helices contains His Pro Asp (HPD) sequence present in all J-domains and required for the stimulation of Hsp70s’ ATPase. (bottom right) Hsp70’s SBD interacting with a peptide derived from a client protein (PDB ID: 1DKZ). Note helical α-SBD subdomain covering the binding cleft of β-SBD, but without contacting the client.