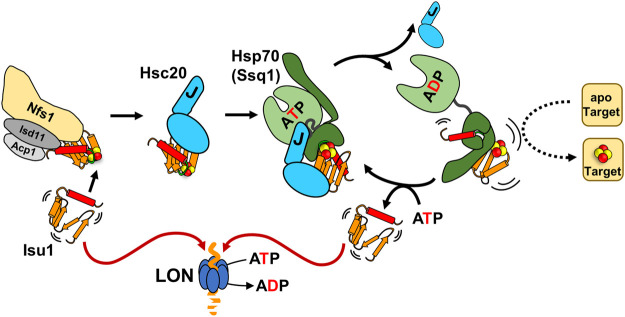
FIGURE 3.
The role of Hsc20/Hsp70 scaffold binding cycle in FeS cluster biogenesis. Interaction of the Isu/IscU scaffold, which is structurally highly dynamic (indicated by agitrons) with a FeS cluster assembly complex (Nfs1/Isd11/Acp1 and other accessory proteins: frataxin, ferredoxin-not shown) is required for de novo cluster synthesis. The cluster loaded scaffold interacts with the dedicated JDP Hsc20. The scaffold interactions with both the cluster assembly complex and Hsc20 protect it from degradation by LON protease. Hsc20-scaffold complex interacts with its Hsp70 partner in the ATP bound conformation. Synergistic stimulation of Hsp70’s ATPase by the J-domain of Hsc20 and the scaffold triggers conformational changes that leads to Hsc20 release and stabilization of an Hsp70-scaffold complex. Formation of this complex is required for effective cluster transfer onto apo-target proteins. Exchange of ADP for ATP results in scaffold release. It is not clear what regulates whether a scaffold is reused in another round of cluster assembly/transfer or proteolyzed.