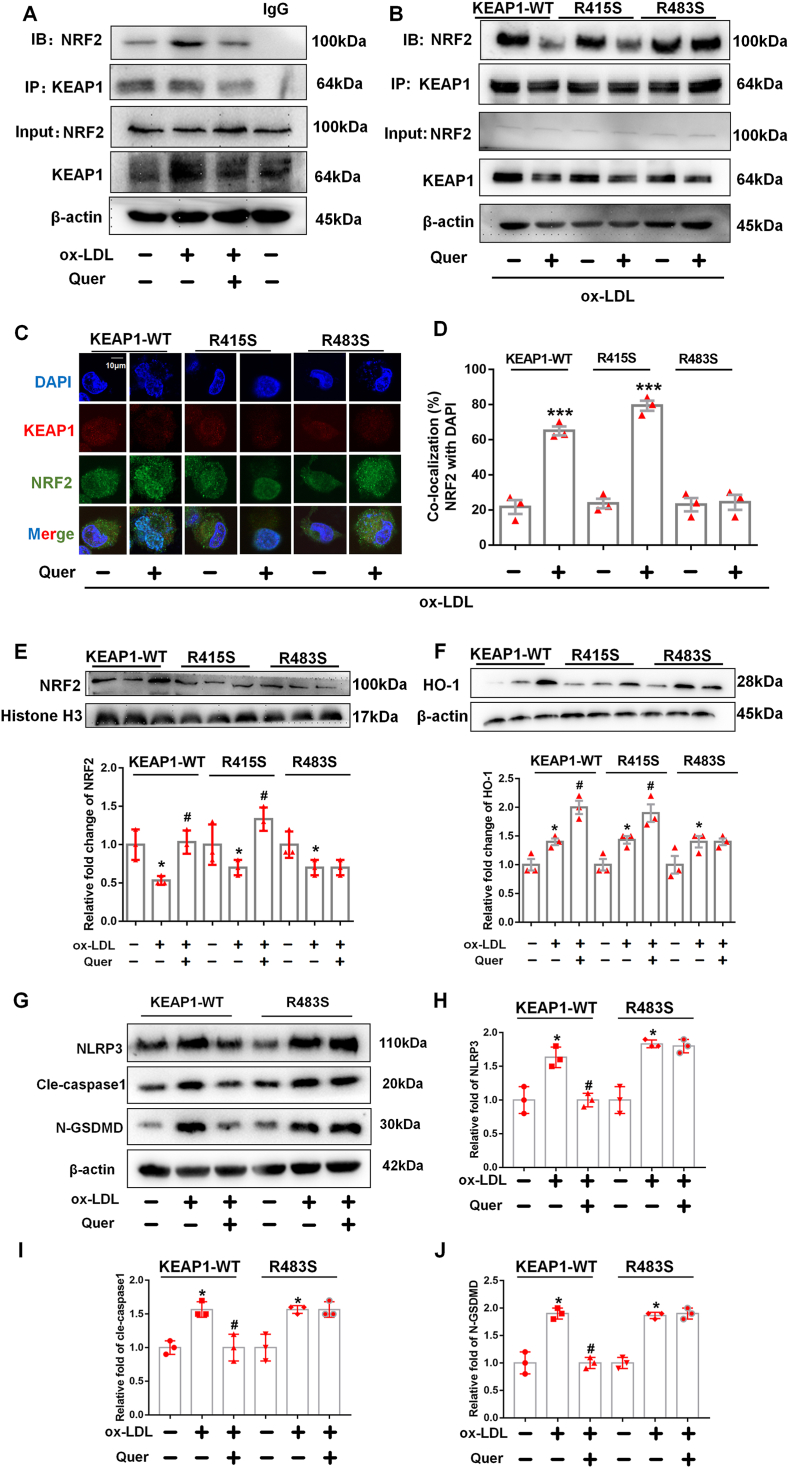
Fig. 8.
Quercetin form hydrogen bond with KEAP1 at Arg483 to regulate NRF2 activity and reduce the generation of ROS in ox-LDL treated macrophages.
Plasmid with mutated KEAP1 at R415S and R483S are transfected into macrophages, followed by quercetin (50 μM) treated for another 1 h. The macrophages lysates are immunoprecipitated with anti-KEAP1 antibody, and the immunoprecipitated proteins are used to immunoblot analysis with anti-NRF2 antibodies. The nuclear lysates are analyzed with anti–HO–1 and anti-histone H3 antibody. Anti-KEAP1 and anti-NRF2 are used for co-localized fluorescent staining. (A). Immunoprecipitation is used to detect the formation of NRF2 and KEAP1 complex (n = 3). (B–D). After quercetin treatment, immunoprecipitation and confocal fluorescent staining are used to detect the formation of NRF2 and KEAP1 complex and nuclear translocation of NRF2 in KEAP1-WT, KEAP1-R415S and KEAP1-R483S mutated macrophages (n = 3, ***p < 0.001, ox-LDL vs Quer + ox-LDL group). (E). Western blot is used to detect the NRF2 level in nuclear lysate (n = 3, *p < 0.05, vs control group. #p < 0.05, vs ox-LDL group). (F). Western blot is used to evaluate the HO-1 level in total lysate (n = 3, *p < 0.05, vs control group. #p < 0.05, vs ox-LDL group).